A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages
- PMID: 28367972
- PMCID: PMC5382263
- DOI: 10.1038/ncomms14967
A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages
Erratum in
-
Corrigendum: A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages.Nat Commun. 2017 May 17;8:15673. doi: 10.1038/ncomms15673. Nat Commun. 2017. PMID: 28513619 Free PMC article. No abstract available.
Abstract
Conditional expression of diphtheria toxin receptor (DTR) is widely used for tissue-specific ablation of cells. However, diphtheria toxin (DT) crosses the blood-brain barrier, which limits its utility for ablating peripheral cells using Cre drivers that are also expressed in the central nervous system (CNS). Here we report the development of a brain-sparing DT, termed BRAINSPAReDT, for tissue-specific genetic ablation of cells outside the CNS. We prevent blood-brain barrier passage of DT through PEGylation, which polarizes the molecule and increases its size. We validate BRAINSPAReDT with regional genetic sympathectomy: BRAINSPAReDT ablates peripheral but not central catecholaminergic neurons, thus avoiding the Parkinson-like phenotype associated with full dopaminergic depletion. Regional sympathectomy compromises adipose tissue thermogenesis, and renders mice susceptible to obesity. We provide a proof of principle that BRAINSPAReDT can be used for Cre/DTR tissue-specific ablation outside the brain using CNS drivers, while consolidating the link between adiposity and the sympathetic nervous system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
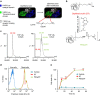
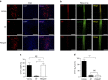

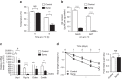

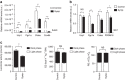
References
-
- Picklo M. J. Methods of sympathetic degeneration and alteration. J. Auton. Nerv. Syst. 62, 111–125 (1997). - PubMed
-
- Beznák A. B. L. & Hasch Z. The effect of sympathectomy on the fatty deposit in connective tissue. Q. J. Exp. Physiol. 27, 5–12 (1937).
-
- Cousin B. et al. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 133, 2255–2262 (1993). - PubMed
-
- Youngstrom T. G. & Bartness T. J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol. 275, 1488–1493 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

