Riboflavin deficiency induces a significant change in proteomic profiles in HepG2 cells
- PMID: 28367977
- PMCID: PMC5377456
- DOI: 10.1038/srep45861
Riboflavin deficiency induces a significant change in proteomic profiles in HepG2 cells
Abstract
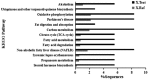
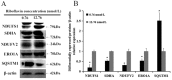
Riboflavin deficiency is widespread in many regions over the world, especially in underdeveloped countries. In this study, we investigated the effects of riboflavin deficiency on protein expression profiles in HepG2 cells in order to provide molecular information for the abnormalities induced by riboflavin deficiency. HepG2 cells were cultured in media containing different concentrations of riboflavin. Changes of cell viability and apoptosis were assessed. A comparative proteomic analysis was performed using a label-free shotgun method with LC-MS/MS to investigate the global changes of proteomic profiles in response to riboflavin deficiency. Immunoblotting test was used to validate the results of proteomic approach. The cell viability and apoptosis tests showed that riboflavin was vital in maintaining the cytoactivity of HepG2 cells. The label-free proteomic analysis revealed that a total of 37 proteins showing differential expression (±2 fold, p < 0.05) were identified after riboflavin deficiency. Bioinformatics analysis indicated that the riboflavin deficiency caused an up-regulation of Parkinson's disease pathway, steroid catabolism, endoplasmic reticulum stress and apoptotic process, while the fatty acid metabolism, tricarboxylic citrate cycle, oxidative phosphorylation and iron metabolism were down-regulated. These findings provide a molecular basis for the elucidation of the effects caused by riboflavin deficiency.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Hoey. L., McNulty H. & Strain J. J. Studies of biomarker responses to intervention with riboflavin: a systematic review. Am J Clin Nutr 89, 1960–1980 (2009). - PubMed
-
- McNulty H. et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C → T polymorphism. Circulation 13, 74–80 (2006). - PubMed
-
- Powers H. J. et al. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am J Clin Nutr 93, 1274–1284 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

