Roles of Oxygen and Hydrogen in Crystal Orientation Transition of Copper Foils for High-Quality Graphene Growth
- PMID: 28367988
- PMCID: PMC5377254
- DOI: 10.1038/srep45358
Roles of Oxygen and Hydrogen in Crystal Orientation Transition of Copper Foils for High-Quality Graphene Growth
Abstract
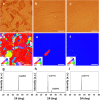
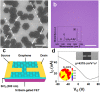
The high-quality graphene film can be grown on single-crystal Cu substrate by seamlessly stitching the aligned graphene domains. The roles of O2 and H2 have been intensively studied in the graphene growth kinetics, including lowering the nucleation sites and tailoring the domain structures. However, how the O2 and H2 influence Cu orientations during recrystallization prior to growing graphene, still remains unclear. Here we report that the oxidation of Cu surface tends to stabilize the Cu(001) orientation while impedes the evolution of Cu(111) single domain during annealing process. The crystal orientation-controlled synthesis of aligned graphene seeds is further realized on the long-range ordered Cu(111) substrate. With decreasing the thickness of oxide layer on Cu surface by introducing H2, the Cu(001) orientation changes into Cu(111) orientation. Meanwhile, the average domain size of Cu foils is increased from 50 μm to larger than 1000 μm. The density functional theory calculations reveal that the oxygen increases the energy barrier for Cu(111) surface and makes O/Cu(001) more stable than O/Cu(111) structure. Our work can be helpful for revealing the roles of O2 and H2 in controlling the formation of Cu single-crystal substrate as well as in growing high-quality graphene films.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Li X. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 324, 1312–1314 (2009). - PubMed
-
- Bae S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotech. 5, 574–578 (2010). - PubMed
-
- Deng B. et al. Roll-to-roll encapsulation of metal nanowires between graphene and plastic substrate for high-performance flexible transparent electrodes. Nano Lett. 15, 4206–4213 (2015). - PubMed
-
- Huang P. et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature. 469, 389–392 (2011). - PubMed
-
- Kim K. et al. Grain boundary mapping in polycrystalline graphene. ACS Nano. 5, 2142–2146 (2011). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

