Mechanisms of central tolerance for B cells
- PMID: 28368006
- PMCID: PMC5623591
- DOI: 10.1038/nri.2017.19
Mechanisms of central tolerance for B cells
Abstract
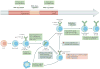
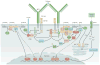
Immune tolerance hinders the potentially destructive responses of lymphocytes to host tissues. Tolerance is regulated at the stage of immature B cell development (central tolerance) by clonal deletion, involving apoptosis, and by receptor editing, which reprogrammes the specificity of B cells through secondary recombination of antibody genes. Recent mechanistic studies have begun to elucidate how these divergent mechanisms are controlled. Single-cell antibody cloning has revealed defects of B cell central tolerance in human autoimmune diseases and in several human immunodeficiency diseases caused by single gene mutations, which indicates the relevance of B cell tolerance to disease and suggests possible genetic pathways that regulate tolerance.
Conflict of interest statement
Competing interests statement
The author declares no competing interests.
Figures


References
-
- Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. A monumental paper that uses immunoglobulin transgenic mice to show that clonal anergy is a mechanism of B cell tolerance. - PubMed
-
- Klinman NR. The “clonal selection hypothesis” and current concepts of B cell tolerance. Immunity. 1996;5:189–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

