Halogen-π Interactions in the Cytochrome P450 Active Site: Structural Insights into Human CYP2B6 Substrate Selectivity
- PMID: 28368100
- PMCID: PMC5748325
- DOI: 10.1021/acschembio.7b00056
Halogen-π Interactions in the Cytochrome P450 Active Site: Structural Insights into Human CYP2B6 Substrate Selectivity
Abstract
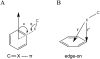
Numerous cytochrome P450 (CYP) 2B6 substrates including drugs and environmental chemicals are halogenated. To assess the role of halogen-π bonds in substrate selectivity and orientation in the active site, structures of four CYP2B6 monoterpenoid complexes were solved by X-ray crystallography. Bornyl bromide exhibited dual orientations in the active site with the predominant orientation revealing a bromine-π bond with the Phe108 side chain. Bornane demonstrated two orientations with equal occupancy; in both, the C2 atom that bears the bromine in bornyl bromide was displaced by more than 2.5 Å compared with the latter complex. The bromine in myrtenyl bromide π-bonded with Phe297 in CYP2B6, whereas the two major orientations in the active site mutant I114V exhibited bromine-π interactions with two additional residues, Phe108 and Phe115. Analysis of existing structures suggests that halogen-π interactions may be unique to the CYP2B enzymes within CYP family 2 but are also important for CYP3A enzymes.
Figures
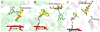


References
-
- Politzer P, Murray JS, Clark T. Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys Chem Chem Phys. 2010;12:7748–57. - PubMed
-
- Liu Y, Xu Z, Yang Z, Chen K, Zhu W. A knowledge-based halogen bonding scoring function for predicting protein-ligand interactions. J Mol Model. 2014;19:5015–30. - PubMed
-
- Wang H, Wang W, Jin WJ. sigma-Hole Bond vs pi-Hole Bond: A Comparison Based on Halogen Bond. Chem Rev. 2016;116:5072–104. - PubMed
-
- Himmel DM, Das K, Clark AD, Jr, Hughes SH, Benjahad A, Oumouch S, Guillemont J, Coupa S, Poncelet A, Csoka I, Meyer C, Andries K, Nguyen CH, Grierson DS, Arnold E. Crystal structures for HIV-1 reverse transcriptase in complexes with three pyridinone derivatives: a new class of non-nucleoside inhibitors effective against a broad range of drug-resistant strains. J Med Chem. 2005;48:7582–91. - PubMed
-
- Rohde LA, Ahring PK, Jensen ML, Nielsen EO, Peters D, Helgstrand C, Krintel C, Harpsoe K, Gajhede M, Kastrup JS, Balle T. Intersubunit bridge formation governs agonist efficacy at nicotinic acetylcholine alpha4beta2 receptors: unique role of halogen bonding revealed. J Biol Chem. 2012;287:4248–59. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

