ICAM-1 Binding Rhinoviruses Enter HeLa Cells via Multiple Pathways and Travel to Distinct Intracellular Compartments for Uncoating
- PMID: 28368306
- PMCID: PMC5408674
- DOI: 10.3390/v9040068
ICAM-1 Binding Rhinoviruses Enter HeLa Cells via Multiple Pathways and Travel to Distinct Intracellular Compartments for Uncoating
Abstract
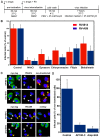
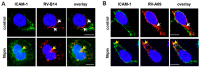
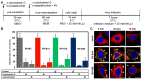
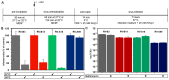
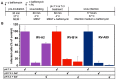
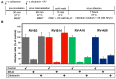
Of the more than 150 human rhinovirus (RV) serotypes, 89 utilize intercellular adhesion molecule-1 (ICAM-1) for cell entry. These belong either to species A or B. We recently demonstrated that RV-B14 and RV-A89, despite binding this same receptor, are routed into distinct endosomal compartments for release of their RNA into the cytosol. To gain insight into the underlying mechanism we now comparatively investigate the port of entry, temperature-dependence of uncoating, and intracellular routing of RV-B3, RV-B14, RV-A16, and RV-A89 in HeLa cells. The effect of various drugs blocking distinct stages on the individual pathways was determined via comparing the number of infected cells in a TissueFaxs instrument. We found that RV-B14 and RV-A89 enter via clathrin-, dynamin-, and cholesterol-dependent pathways, as well as by macropinocytosis. Drugs interfering with actin function similarly blocked entry of all four viruses, indicating their dependence on a dynamic actin network. However, uniquely, RV-A89 was able to produce progeny when internalized at 20 °C followed by neutralizing the endosomal pH and further incubation at 37 °C. Blocking dynein-dependent endosomal transport prevented uncoating of RV-A16 and RV-A89, but not of RV-B3 and RV-B14, indicative for routing of RV-A16 and RV-A89 into the endocytic recycling compartment for uncoating. Our results call for caution when developing drugs aimed at targeting entry or intracellular trafficking of all rhinovirus serotypes.
Keywords: ICAM-1; actin; clathrin; dynamin; dynein; endocytosis; human rhinoviruses; macropinocytosis; productive uncoating.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
ICAM-1 Binding Rhinoviruses A89 and B14 Uncoat in Different Endosomal Compartments.J Virol. 2016 Aug 12;90(17):7934-42. doi: 10.1128/JVI.00712-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334586 Free PMC article.
-
Chemical Evolution of Rhinovirus Identifies Capsid-Destabilizing Mutations Driving Low-pH-Independent Genome Uncoating.J Virol. 2022 Jan 26;96(2):e0106021. doi: 10.1128/JVI.01060-21. Epub 2021 Oct 27. J Virol. 2022. PMID: 34705560 Free PMC article.
-
Entry of human rhinovirus 89 via ICAM-1 into HeLa epithelial cells is inhibited by actin skeleton disruption and by bafilomycin.Arch Virol. 2014 Jan;159(1):125-40. doi: 10.1007/s00705-013-1797-1. Epub 2013 Aug 4. Arch Virol. 2014. PMID: 23913188
-
Uncoating of human rhinoviruses.Rev Med Virol. 2010 Sep;20(5):281-97. doi: 10.1002/rmv.654. Rev Med Virol. 2010. PMID: 20629045 Review.
-
Viral cell recognition and entry.Protein Sci. 1994 Oct;3(10):1712-25. doi: 10.1002/pro.5560031010. Protein Sci. 1994. PMID: 7849588 Free PMC article. Review.
Cited by
-
Impairing the function of MLCK, myosin Va or myosin Vb disrupts Rhinovirus B14 replication.Sci Rep. 2017 Dec 7;7(1):17153. doi: 10.1038/s41598-017-17501-z. Sci Rep. 2017. PMID: 29215055 Free PMC article.
-
Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens.Viruses. 2025 Feb 12;17(2):252. doi: 10.3390/v17020252. Viruses. 2025. PMID: 40007007 Free PMC article. Review.
-
A synthetic curcumin-like diarylpentanoid analog inhibits rhinovirus infection in H1 hela cells via multiple antiviral mechanisms.Daru. 2024 Dec;32(2):729-744. doi: 10.1007/s40199-024-00542-x. Epub 2024 Oct 12. Daru. 2024. PMID: 39395148
-
Plasmolipin regulates basolateral-to-apical transcytosis of ICAM-1 and leukocyte adhesion in polarized hepatic epithelial cells.Cell Mol Life Sci. 2022 Jan 9;79(1):61. doi: 10.1007/s00018-021-04095-z. Cell Mol Life Sci. 2022. PMID: 34999972 Free PMC article.
-
Lactoferrin affects rhinovirus B-14 entry into H1-HeLa cells.Arch Virol. 2021 Apr;166(4):1203-1211. doi: 10.1007/s00705-021-04993-4. Epub 2021 Feb 19. Arch Virol. 2021. PMID: 33606112 Free PMC article.
References
-
- Vlasak M., Roivainen M., Reithmayer M., Goesler I., Laine P., Snyers L., Hovi T., Blaas D. The minor receptor group of human rhinovirus (HRV) includes HRV23 and HRV25, but the presence of a lysine in the VP1 HI loop is not sufficient for receptor binding. J. Virol. 2005;79:7389–7395. doi: 10.1128/JVI.79.12.7389-7395.2005. - DOI - PMC - PubMed
-
- McErlean P., Shackelton L.A., Andrews E., Webster D.R., Lambert S.B., Nissen M.D., Sloots T.P., Mackay I.M. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS ONE. 2008;3:e1847. doi: 10.1371/journal.pone.0001847. - DOI - PMC - PubMed
-
- Bochkov Y.A., Watters K., Ashraf S., Griggs T.F., Devries M.K., Jackson D.J., Palmenberg A.C., Gern J.E. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl. Acad. Sci. USA. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

