CUL-2LRR-1 and UBXN-3 drive replisome disassembly during DNA replication termination and mitosis
- PMID: 28368371
- PMCID: PMC5410169
- DOI: 10.1038/ncb3500
CUL-2LRR-1 and UBXN-3 drive replisome disassembly during DNA replication termination and mitosis
Abstract
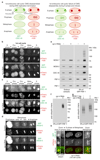
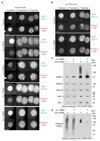
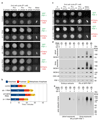
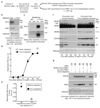
Replisome disassembly is the final step of DNA replication in eukaryotes, involving the ubiquitylation and CDC48-dependent dissolution of the CMG helicase (CDC45-MCM-GINS). Using Caenorhabditis elegans early embryos and Xenopus laevis egg extracts, we show that the E3 ligase CUL-2LRR-1 associates with the replisome and drives ubiquitylation and disassembly of CMG, together with the CDC-48 cofactors UFD-1 and NPL-4. Removal of CMG from chromatin in frog egg extracts requires CUL2 neddylation, and our data identify chromatin recruitment of CUL2LRR1 as a key regulated step during DNA replication termination. Interestingly, however, CMG persists on chromatin until prophase in worms that lack CUL-2LRR-1, but is then removed by a mitotic pathway that requires the CDC-48 cofactor UBXN-3, orthologous to the human tumour suppressor FAF1. Partial inactivation of lrr-1 and ubxn-3 leads to synthetic lethality, suggesting future approaches by which a deeper understanding of CMG disassembly in metazoa could be exploited therapeutically.
Conflict of interest statement
The authors confirm that they have no competing financial interests.
Figures



Comment in
-
Two paths to let the replisome go.Cell Death Differ. 2017 Jul;24(7):1140-1141. doi: 10.1038/cdd.2017.75. Epub 2017 May 19. Cell Death Differ. 2017. PMID: 28524853 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

