Wnt signaling in triple-negative breast cancer
- PMID: 28368389
- PMCID: PMC5520491
- DOI: 10.1038/oncsis.2017.14
Wnt signaling in triple-negative breast cancer
Abstract
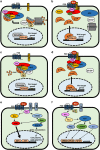
Wnt signaling regulates a variety of cellular processes, including cell fate, differentiation, proliferation and stem cell pluripotency. Aberrant Wnt signaling is a hallmark of many cancers. An aggressive subtype of breast cancer, known as triple-negative breast cancer (TNBC), demonstrates dysregulation in canonical and non-canonical Wnt signaling. In this review, we summarize regulators of canonical and non-canonical Wnt signaling, as well as Wnt signaling dysfunction that mediates the progression of TNBC. We review the complex molecular nature of TNBC and the emerging therapies that are currently under investigation for the treatment of this disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

