Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma
- PMID: 28368412
- PMCID: PMC5558095
- DOI: 10.1038/onc.2017.91
Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma
Abstract
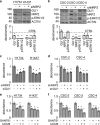
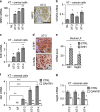
Aberrant Hedgehog/GLI signaling has been implicated in a diverse spectrum of human cancers, but its role in lung adenocarcinoma (LAC) is still under debate. We show that the downstream effector of the Hedgehog pathway, GLI1, is expressed in 76% of LACs, but in roughly half of these tumors, the canonical pathway activator, Smoothened, is expressed at low levels, possibly owing to epigenetic silencing. In LAC cells including the cancer stem cell compartment, we show that GLI1 is activated noncanonically by MAPK/ERK signaling. Different mechanisms can trigger the MAPK/ERK/GLI1 cascade including KRAS mutation and stimulation of NRP2 by VEGF produced by the cancer cells themselves in an autocrine loop or by stromal cells as paracrine cross talk. Suppression of GLI1, by silencing or drug-mediated, inhibits LAC cells proliferation, attenuates their stemness and increases their susceptibility to apoptosis in vitro and in vivo. These findings provide insight into the growth of LACs and point to GLI1 as a downstream effector for oncogenic pathways. Thus, strategies involving direct inhibition of GLI1 may be useful in the treatment of LACs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Targeting GLI1 Suppresses Cell Growth and Enhances Chemosensitivity in CD34+ Enriched Acute Myeloid Leukemia Progenitor Cells.Cell Physiol Biochem. 2016;38(4):1288-302. doi: 10.1159/000443075. Epub 2016 Mar 24. Cell Physiol Biochem. 2016. PMID: 27008269
-
GLI1 promotes cancer stemness through intracellular signaling pathway PI3K/Akt/NFκB in colorectal adenocarcinoma.Exp Cell Res. 2018 Dec 15;373(1-2):145-154. doi: 10.1016/j.yexcr.2018.10.006. Epub 2018 Oct 12. Exp Cell Res. 2018. PMID: 30321514
-
IQGAP3 activates Hedgehog signaling to confer stemness and metastasis via up-regulating GLI1 in lung cancer.Sci Rep. 2024 Dec 28;14(1):31327. doi: 10.1038/s41598-024-82793-x. Sci Rep. 2024. PMID: 39732803 Free PMC article.
-
GLI1, a master regulator of the hallmark of pancreatic cancer.Pathol Int. 2016 Dec;66(12):653-660. doi: 10.1111/pin.12476. Epub 2016 Nov 8. Pathol Int. 2016. PMID: 27862693 Review.
-
Role of Glioma-associated GLI1 Oncogene in Carcinogenesis and Cancertargeted Therapy.Curr Cancer Drug Targets. 2018;18(6):558-566. doi: 10.2174/1568009618666171129223533. Curr Cancer Drug Targets. 2018. PMID: 29189160 Review.
Cited by
-
Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance.Cancers (Basel). 2022 Jan 13;14(2):381. doi: 10.3390/cancers14020381. Cancers (Basel). 2022. PMID: 35053542 Free PMC article. Review.
-
N6-methyladenine-mediated aberrant activation of the lncRNA SOX2OT-GLI1 loop promotes non-small-cell lung cancer stemness.Cell Death Discov. 2023 May 6;9(1):149. doi: 10.1038/s41420-023-01442-w. Cell Death Discov. 2023. PMID: 37149646 Free PMC article.
-
Gli1-mediated tumor cell-derived bFGF promotes tumor angiogenesis and pericyte coverage in non-small cell lung cancer.J Exp Clin Cancer Res. 2024 Mar 16;43(1):83. doi: 10.1186/s13046-024-03003-0. J Exp Clin Cancer Res. 2024. PMID: 38493151 Free PMC article.
-
Sodium Butyrate Combined with Docetaxel for the Treatment of Lung Adenocarcinoma A549 Cells by Targeting Gli1.Onco Targets Ther. 2020 Sep 4;13:8861-8875. doi: 10.2147/OTT.S252323. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982280 Free PMC article.
-
Glycolytic Metabolic Remodeling by the Truncate of Glioma-Associated Oncogene Homolog 1 in Triple-Negative Breast Cancer Cells.J Cancer. 2022 Aug 8;13(10):3031-3043. doi: 10.7150/jca.72793. eCollection 2022. J Cancer. 2022. PMID: 36046646 Free PMC article.
References
-
- Lee RTH, Zhao Z, Ingham PW. Hedgehog signalling. Development 2016; 143: 367–372. - PubMed
-
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev 2008; 22: 2454–2472. - PubMed
-
- Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 2009; 9: 873–886. - PubMed
-
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O'Brien SJ et al. Identification of an amplified, highly expressed gene in a human glioma. Science 1987; 236: 70–73. - PubMed
-
- Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RC et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 2005; 24: 4232–4242. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

