Enhanced Glycolytic Metabolism Contributes to Cardiac Dysfunction in Polymicrobial Sepsis
- PMID: 28368517
- PMCID: PMC5451607
- DOI: 10.1093/infdis/jix138
Enhanced Glycolytic Metabolism Contributes to Cardiac Dysfunction in Polymicrobial Sepsis
Abstract
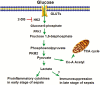
Background: Cardiac dysfunction is present in >40% of sepsis patients and is associated with mortality rates of up to 70%. Recent evidence suggests that glycolytic metabolism plays a critical role in host defense and inflammation. Activation of Toll-like receptors on immune cells can enhance glycolytic metabolism. This study investigated whether modulation of glycolysis by inhibition of hexokinase will be beneficial to septic cardiomyopathy.
Methods: Male C57B6/J mice were treated with a hexokinase inhibitor (2-deoxy-d-glucose [2-DG], 0.25-2 g/kg, n = 6-8) before cecal ligation and puncture (CLP) induced sepsis. Untreated septic mice served as control. Sham surgically operated mice treated with or without the 2-DG inhibitor served as sham controls. Cardiac function was assessed 6 hours after CLP sepsis by echocardiography. Serum was harvested for measurement of inflammatory cytokines and lactate.
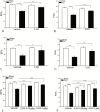
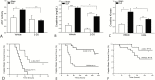
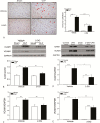
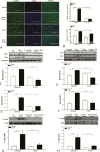
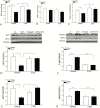
Results: Sepsis-induced cardiac dysfunction was significantly attenuated by administration of 2-DG. Ejection fraction and fractional shortening in 2-DG-treated septic mice were significantly (P < .05) greater than in untreated CLP mice. 2-DG administration also significantly improved survival outcome, reduced kidney and liver injury, attenuated sepsis-increased serum levels of tumor necrosis factor α and interleukin 1β as well as lactate, and enhanced the expression of Sirt1 and Sirt3 in the myocardium, which play an important role in mitochondrial function and metabolism. In addition, 2-DG administration suppresses sepsis-increased expression of apoptotic inducers Bak and Bax as well as JNK phosphorylation in the myocardium.
Conclusions: Glycolytic metabolism plays an important role in mediating sepsis-induced septic cardiomyopathy. The mechanisms may involve regulation of inflammatory response and apoptotic signaling.
Keywords: 2-deoxy-D-glucose; cardiomyopathy; glycolysis; inflammatory responses.; sepsis.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

