Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children - A randomized controlled trial
- PMID: 28368738
- PMCID: PMC5489287
- DOI: 10.1080/21645515.2017.1287640
Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children - A randomized controlled trial
Erratum in
-
Correction.Hum Vaccin Immunother. 2021 Mar 4;17(3):928-930. doi: 10.1080/21645515.2020.1760708. Epub 2020 Jul 21. Hum Vaccin Immunother. 2021. PMID: 32693672 Free PMC article. No abstract available.
Abstract
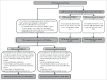
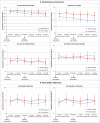
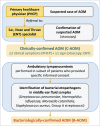
We previously reported 10-valent pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV) efficacy in a double-blind randomized trial (ClinicalTrials.gov: NCT00466947) against various diseases, including acute otitis media (AOM). Here, we provide further analyses. In the Panamanian subset, 7,359 children were randomized (1:1) to receive PHiD-CV or control vaccine at age 2/4/6 and 15-18 months. Of these, 2,000 had nasopharyngeal swabs collected. AOM cases were captured when parents sought medical attention for children with AOM symptoms; surveillance was enhanced approximately 2 y into the study through regular telephone calls or home visits by study personnel, who advised parents to visit the clinic if their child had AOM symptoms. Mean follow-up was 31.4 months. Clinical AOM (C-AOM) cases were assessed by physicians and confirmed by otorhinolaryngologists. Middle ear fluid samples, taken from children with C-AOM after specific informed consent, and nasopharyngeal samples were cultured for pathogen identification. For 7,359 children, 2,574 suspected AOM cases were assessed by a primary healthcare physician; 649 cases were C-AOM cases as per protocol definition. From the 503 MEF samples collected, 158 resulted in a positive culture. In the intent-to-treat cohort (7,214 children), PHiD-CV showed VE against first C-AOM (24.0% [95% CI: 8.7, 36.7]) and bacterial (B-AOM) episodes (48.0% [20.3, 66.1]) in children <24 months, which declined thereafter with age. Pre-booster VE against C-AOM was 30.7% [12.9, 44.9]; post-booster, -6.7% [-36.4, 16.6]. PHiD-CV VE was 17.7% [-6.1, 36.2] against moderate and 32.7% [-20.5, 62.4] against severe C-AOM. VE against vaccine-serotype pneumococcal NPC was 31.2% [5.3, 50.3] 3 months post-booster, and 25.6% [12.7, 36.7] across all visits. NTHi colonization rates were low and no significant reduction was observed. PHiD-CV showed efficacy against C-AOM and B-AOM in children younger than 24 months, and reduced vaccine-serotype NPC.
Keywords: acute otitis media; children; efficacy; nasopharyngeal carriage; pneumococcal conjugate vaccination.
Figures



References
-
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 2012; 7: e36226; PMID:22558393; https://doi.org/ 10.1371/journal.pone.0036226 - DOI - PMC - PubMed
-
- Cripps AW, Otczyk DC, Kyd JM. Bacterial otitis media: a vaccine preventable disease? Vaccine 2005; 23: 2304-10; PMID:15755616; https://doi.org/ 10.1016/j.vaccine.2005.01.023 - DOI - PubMed
-
- Klein JO. Otitis media. Clin Infect Dis 1994; 19: 823-33; PMID:7893865; https://doi.org/ 10.1093/clinids/19.5.823 - DOI - PubMed
-
- Vergison A, Dagan R, Arguedas A, Bonhoeffer J, Cohen R, Dhooge I, Hoberman A, Liese J, Marchisio P, Palmu AA, et al.. Otitis media and its consequences: beyond the earache. Lancet Infect Dis 2010; 10: 195-203; PMID:20185098; https://doi.org/ 10.1016/S1473-3099(10)70012-8 - DOI - PubMed
-
- Bardach A, Ciapponi A, Garcia-Marti S, Glujovsky D, Mazzoni A, Fayad A, Colindres RE, Gentile A. Epidemiology of acute otitis media in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 2011; 75: 1062-70; PMID:21665297; https://doi.org/ 10.1016/j.ijporl.2011.05.014 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
