Polarization and migration in the zebrafish posterior lateral line system
- PMID: 28369079
- PMCID: PMC5393887
- DOI: 10.1371/journal.pcbi.1005451
Polarization and migration in the zebrafish posterior lateral line system
Abstract
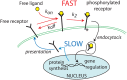
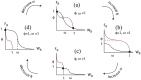
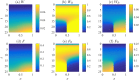
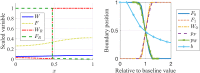
Collective cell migration plays an important role in development. Here, we study the posterior lateral line primordium (PLLP) a group of about 100 cells, destined to form sensory structures, that migrates from head to tail in the zebrafish embryo. We model mutually inhibitory FGF-Wnt signalling network in the PLLP and link tissue subdivision (Wnt receptor and FGF receptor activity domains) to receptor-ligand parameters. We then use a 3D cell-based simulation with realistic cell-cell adhesion, interaction forces, and chemotaxis. Our model is able to reproduce experimentally observed motility with leading cells migrating up a gradient of CXCL12a, and trailing (FGF receptor active) cells moving actively by chemotaxis towards FGF ligand secreted by the leading cells. The 3D simulation framework, combined with experiments, allows an investigation of the role of cell division, chemotaxis, adhesion, and other parameters on the shape and speed of the PLLP. The 3D model demonstrates reasonable behaviour of control as well as mutant phenotypes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

