Glial Calcium Waves are Triggered by Seizure Activity and Not Essential for Initiating Ictal Onset or Neurovascular Coupling
- PMID: 28369176
- PMCID: PMC6433182
- DOI: 10.1093/cercor/bhx072
Glial Calcium Waves are Triggered by Seizure Activity and Not Essential for Initiating Ictal Onset or Neurovascular Coupling
Abstract
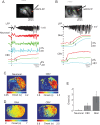
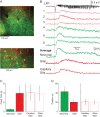
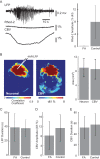
It has been postulated that glia play a critical role in modifying neuronal activity, mediating neurovascular coupling, and in seizure initiation. We investigated the role of glia in ictogenesis and neurovascular coupling through wide-field multicell and 2-photon single cell imaging of calcium and intrinsic signal imaging of cerebral blood volume in an in vivo rat model of focal neocortical seizures. Ictal events triggered a slowly propagating glial calcium wave that was markedly delayed after both neuronal and hemodynamic onset. Glial calcium waves exhibited a stereotypical spread that terminated prior to seizure offset and propagated to an area ~60% greater than the propagation area of neural and vascular signals. Complete blockage of glial activity with fluoroacetate resulted in no change in either neuronal or hemodynamic activity. These ictal glial waves were blocked by carbenoxolone, a gap junction blocker. Our in vivo data reveal that ictal events trigger a slowly propagating, stereotypical glial calcium wave, mediated by gap junctions, that is spatially and temporally independent of neuronal and hemodynamic activities. We introduce a novel ictally triggered propagating glial calcium wave calling into question the criticality of glial calcium wave in both ictal onset and neurovascular coupling.
Keywords: epilepsy; glia; ictogenesis; neurovascular coupling; optical imaging.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Bazargani N, Attwell D. 2016. Astrocyte calcium signaling: the third wave. Nat Neurosci. 19:182–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

