Limitations of Rapid Diagnostic Testing in Patients with Suspected Malaria: A Diagnostic Accuracy Evaluation from Swaziland, a Low-Endemicity Country Aiming for Malaria Elimination
- PMID: 28369268
- PMCID: PMC5399938
- DOI: 10.1093/cid/cix131
Limitations of Rapid Diagnostic Testing in Patients with Suspected Malaria: A Diagnostic Accuracy Evaluation from Swaziland, a Low-Endemicity Country Aiming for Malaria Elimination
Abstract
Background: The performance of Plasmodium falciparum-specific histidine-rich protein 2-based rapid diagnostic tests (RDTs) to evaluate suspected malaria in low-endemicity settings has not been well characterized.
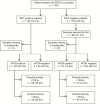
Methods: Using dried blood spot samples from patients with suspected malaria at 37 health facilities from 2012 to 2014 in the low-endemicity country of Swaziland, we investigated the diagnostic accuracy of histidine-rich protein 2-based RDTs using qualitative polymerase chain reaction (PCR) (nested PCR targeting the cytochrome b gene) and quantitative PCR as reference standards. To explore reasons for false-negative and/or false-positive results, we used pfhrp2/3-specific PCR and logistic regression analyses of potentially associated epidemiological factors.
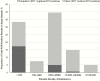
Results: From 1353 patients, 93.0% of RDT-positive (n = 185) and 31.2% of RDT-negative samples (n = 340) were available and selected for testing. Compared with nested PCR, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of RDTs were 51.7%, 94.1%, 67.3%, and 89.1%, respectively. After exclusion of samples with parasite densities <100/μL, which accounted for 75.7% of false-negative results and 33.3% of PCR-detectable infections, the sensitivity, specificity, PPV, and NPV were 78.8%, 93.7%, 62.3%, and 97.1%. Deletions of pfhrp2 were not detected. False-positivity was more likely during the second year and was not associated with demographics, recent malaria, health facility testing characteristics, or potential DNA degradation.
Conclusions: In the low-transmission setting of Swaziland, we demonstrated low sensitivity of RDT for malaria diagnosis, owing to an unexpectedly high proportion of low-density infection among symptomatic subjects. The PPV was also low, requiring further investigation. A more accurate point-of-care diagnostic may be needed to support malaria elimination efforts.
Keywords: Rapid Diagnostic Test; diagnostic accuracy; low transmission; malaria; subpatent infection.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Performance of Rapid Diagnostic Testing in Patients with Suspected Malaria in Cambodia, a Low-Endemicity Country Aiming for Malaria Elimination.Clin Infect Dis. 2017 Oct 30;65(10):1769-1770. doi: 10.1093/cid/cix625. Clin Infect Dis. 2017. PMID: 29020315 No abstract available.
-
Reply to Rossi et al.Clin Infect Dis. 2017 Oct 30;65(10):1770-1771. doi: 10.1093/cid/cix627. Clin Infect Dis. 2017. PMID: 29020318 Free PMC article. No abstract available.
References
-
- World Health Organisation. WHO Global Malaria Programme, World Malaria Report 2015. Geneva, Switzerland: Available at: http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf. Accessed 18 December 2016.
-
- Roll Back Malaria Partnership. Progress & impact series: malaria funding & resource utilization—the first decade of roll back malaria (2010). Available at: http://www.rbm.who.int/ProgressImpactSeries/docs/RBMMalariaFinancingRepo... Accessed 18 December 2016.
-
- Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol 2006; 4(suppl 9):S7–20. - PubMed
-
- World Health Organization. Malaria Rapid Diagnostic Test Performance, Results of WHO product testing of malaria RDTs: round 4. Geneva, Switzerland: World Health Organization, 2012. Available at: http://www.who.int/malaria/publications/rapid_diagnostic/en/. Accessed 18 December 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

