Occipital White Matter Tracts in Human and Macaque
- PMID: 28369290
- PMCID: PMC5890896
- DOI: 10.1093/cercor/bhx070
Occipital White Matter Tracts in Human and Macaque
Abstract
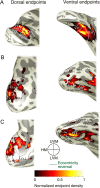
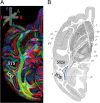
We compare several major white-matter tracts in human and macaque occipital lobe using diffusion magnetic resonance imaging. The comparison suggests similarities but also significant differences in the tracts. There are several apparently homologous tracts in the 2 species, including the vertical occipital fasciculus (VOF), optic radiation, forceps major, and inferior longitudinal fasciculus (ILF). There is one large human tract, the inferior fronto-occipital fasciculus, with no corresponding fasciculus in macaque. We could identify the macaque VOF (mVOF), which has been little studied. Its position is consistent with classical invasive anatomical studies by Wernicke. VOF homology is supported by similarity of the endpoints in V3A and ventral V4 across species. The mVOF fibers intertwine with the dorsal segment of the ILF, but the human VOF appears to be lateral to the ILF. These similarities and differences between the occipital lobe tracts will be useful in establishing which circuitry in the macaque can serve as an accurate model for human visual cortex.
Keywords: comparative study; diffusion MRI; vertical occipital fasciculus; visual cortex; white matter.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Bailey P, Von Bonin G, Davis EW, Garol HW, Mcculloch WS. 1944. Further observations on associational pathways in the brain of macaca mulatta. J Neuropathol Exp Neurol. 3:413.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

