Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study
- PMID: 28369340
- PMCID: PMC5400074
- DOI: 10.1093/eurheartj/ehw593
Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study
Abstract
Aims: To determine tolerability and the optimal dose regimen of the soluble guanylate cyclase stimulator vericiguat in patients with chronic heart failure and preserved ejection fraction (HFpEF).
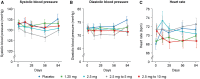
Methods and results: SOCRATES-PRESERVED was a prospective, randomized, placebo-controlled double-blind, Phase 2b dose-finding study in patients with HFpEF (ejection fraction ≥ 45%). Patients received vericiguat once daily at 1.25 or 2.5 mg fixed doses, or 5 or 10 mg titrated from a 2.5 mg starting dose, or placebo for 12 weeks. The two primary endpoints were change from baseline in log-transformed N-terminal pro-B-type natriuretic peptide (NT-ProBNP) and left atrial volume (LAV) at 12 weeks. Patients (N = 477; 48% women; mean age 73 ± 10 years; baseline atrial fibrillation 40%) were randomized within 4 weeks of HF hospitalization (75%) or outpatient treatment with intravenous diuretics for HF (25%) to vericiguat (n = 384) or placebo (n = 93). In the pooled three highest dose arms change in logNT-proBNP (vericiguat: +0.038 ± 0.782 log(pg/mL), n = 195; placebo: -0.098 ± 0.778 log(pg/mL), n = 73; one-sided P = 0.8991, two-sided P = 0.2017), and change in LAV [vericiguat: -1.7 ± 12.8 mL (n = 194); placebo: -3.4 ± 12.7 mL (n = 67), one-sided P = 0.8156, two-sided P = 0.3688] were not different from placebo. Vericiguat was well tolerated (adverse events: vericiguat 10 mg arm, 69.8%; placebo, 73.1%), with low discontinuation rates in all groups, and no changes in blood pressure at 10 mg compared with placebo. The pre-specified exploratory endpoint of Kansas City Cardiomyopathy Questionnaire Clinical Summary Score improved in the vericiguat 10 mg arm by mean 19.3 ± 16.3 points [median 19.8 (interquartile range 10.4-30.7)] from baseline (mean difference from placebo 9.2 points).
Conclusion: Vericiguat was well tolerated, did not change NT-proBNP and LAV at 12 weeks compared with placebo but was associated with improvements in quality of life in patients with HFpEF. Given the encouraging results on quality of life, the effects of vericiguat in patients with HFpEF warrant further study, possibly with higher doses, longer follow-up and additional endpoints.
Keywords: Heart failure with preserved ejection fraction; Soluble guanylate cyclase stimulator; Vericiguat.
© The Author 2016; Published on behalf of the European Society of Cardiology.
Figures


Comment in
-
What can we learn from SOCRATES: more questions than answers?Eur Heart J. 2017 Apr 14;38(15):1128-1131. doi: 10.1093/eurheartj/ehw561. Eur Heart J. 2017. PMID: 28204466 No abstract available.
References
-
- van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ.. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. - PubMed
-
- Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M.. NO-independent regulatory site on soluble guanylate cyclase. Nature 2001;410:212–215. - PubMed
-
- Butler J, Braunwald E, Gheorghiade M.. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA 2014;312:789–790. - PubMed
-
- Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'connor CM, Sun JL, Yancy CW, Young JB.. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

