Single Units in the Posterior Parietal Cortex Encode Patterns of Bimanual Coordination
- PMID: 28369392
- PMCID: PMC5907348
- DOI: 10.1093/cercor/bhx052
Single Units in the Posterior Parietal Cortex Encode Patterns of Bimanual Coordination
Abstract
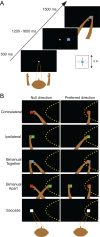
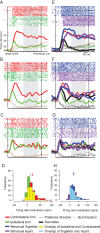
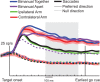
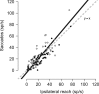
Bimanual coordination is critical for a broad array of behaviors. Drummers, for example, must carefully coordinate movements of their 2 arms, sometimes beating on the same drum and sometimes on different ones. While coordinated behavior is well-studied, the early stages of planning are not well understood. In the parietal reach region (PRR) of the posterior parietal cortex (PPC), the presence of neurons that modulate when either arm moves by itself has been taken as evidence for a role in bimanual coordination. To test this notion, we recorded neurons during both unilateral and bimanual movements. We find that the activity that precedes an ipsilateral arm movement is primarily a sensory response to a target in the neuron's visual receptive field and not a plan to move the ipsilateral arm. In contrast, the activity that precedes a contralateral arm movement is the sum of a movement plan plus a sensory response. Despite not coding ipsilateral arm movements, about half of neurons discriminate between different patterns of bimanual movements. These results provide direct evidence that PRR neurons represent bimanual reach plans, and suggest that bimanual coordination originates in the sensory-to-motor processing stream prior to the motor cortex, within the PPC.
Figures


References
-
- Batista AP, Andersen RA. 2001. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol. 85:539–544. - PubMed
-
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. 2001. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex. 11:528–544. - PubMed
-
- Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B, Genovesio A, Onorati P, Lacquaniti F, Caminiti R. 2000. Early coding of reaching in the parietooccipital cortex. J Neurophysiol. 83:2374–2391. - PubMed
-
- Battaglia-Mayer A, Ferrari-Toniolo S, Visco-Comandini F, Archambault PS, Saberi-Moghadam S, Caminiti R. 2013. Impairment of online control of hand and eye movements in a monkey model of optic ataxia. Cereb Cortex. 23:2644–2656. - PubMed
-
- Breveglieri R, Kutz DF, Fattori P, Gamberini M, Galletti C. 2002. Somatosensory cells in the parieto-occipital area V6A of the macaque. Neuroreport. 13:2113–2116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

