The complex character of photosynthesis in cucumber fruit
- PMID: 28369547
- PMCID: PMC5441898
- DOI: 10.1093/jxb/erx034
The complex character of photosynthesis in cucumber fruit
Abstract
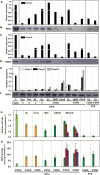
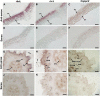
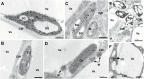
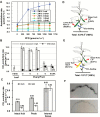
The surface area of a mature green cucumber (Cucumis sativa L.) fruit is comparable with that of a functional leaf, but the characteristics of fruit photosynthesis and its contribution to growth are poorly understood. Here, the photosynthetic properties of two genotypes of cucumber (dark green and light green fruits) were studied using a combination of electron microscopy, immunogold enzyme localization, chlorophyll fluorescence imaging, isotope tracer, and fruit darkening techniques. Chlorophyll content of the exocarp is similar to that of leaves, but there are no distinctive palisade and spongy tissues. The efficiency of PSII is similar to that in leaves, but with lower non-photochemical quenching (NPQ). Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is found mainly in the exocarp, while phosphoenolpyruvate carboxylase (PEPC) is primarily localized to vascular bundles and placenta tissue. Rubisco and PEPC expression at both transcriptional and translational levels increases concurrently during fruit growth. The contribution of fruit photosynthesis in exocarp to its own C accumulation is 9.4%, while ~88% of respiratory CO2 in fruit was captured and re-fixed. Photosynthesis by cucumber fruits, through direct fixation of atmospheric CO2 and recapture of respired CO2, as verified by 14CO2 uptake and gas exchange, makes an important contribution to fruit growth.
Keywords: Chloroplast; PEPC (phosphoenolpyruvate carboxylase); Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase).; cucumber; fruit photosynthesis; respiration.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Aschan G, Pfanz H. 2003. Non-foliar photosynthesis—a strategy of additional carbon acquisition. Flora 198, 81–97.
-
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113. - PubMed
-
- Blanke MM, Lenz F. 1989. Fruit photosynthesis. Plant, Cell and Environment 12, 31–46.
-
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

