NETWORKED 3B: a novel protein in the actin cytoskeleton-endoplasmic reticulum interaction
- PMID: 28369569
- PMCID: PMC5441911
- DOI: 10.1093/jxb/erx047
NETWORKED 3B: a novel protein in the actin cytoskeleton-endoplasmic reticulum interaction
Abstract
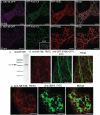
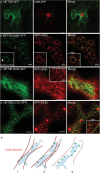
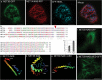
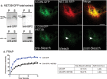
In plants movement of the endoplasmic reticulum (ER) is dependent on the actin cytoskeleton. However little is known about proteins that link the ER membrane and the actin cytoskeleton. Here we identified a novel protein, NETWORKED 3B (NET3B), which is associated with the ER and actin cytoskeleton in vivo. NET3B belongs to a superfamily of plant specific actin binding proteins, the NETWORKED family. NET3B associates with the actin cytoskeleton in vivo through an N-terminal NET actin binding (NAB) domain, which has been well-characterized in other members of the NET family. A three amino acid insertion, Val-Glu-Asp, in the NAB domain of NET3B appears to lower its ability to localize to the actin cytoskeleton compared with NET1A, the founding member of the NET family. The C-terminal domain of NET3B links the protein to the ER. Overexpression of NET3B enhanced the association between the ER and the actin cytoskeleton, and the extent of this association was dependent on the amount of NET3B available. Another effect of NET3B overexpression was a reduction in ER membrane diffusion. In conclusion, our results revealed that NET3B modulates ER and actin cytoskeleton interactions in higher plants.
Keywords: Actin cytoskeleton; N. benthamiana.; NET superfamily; endomembrane system; endoplasmic reticulum.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


References
-
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. 1998. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. The Plant Journal 15, 441–447. - PubMed
-
- Chen J, Stefano G, Brandizzi F, Zheng H. 2011. Arabidopsis RHD3 mediates the generation of the tubular ER network and is required for Golgi distribution and motility in plant cells. Journal of Cell Science 124, 2241–2252. - PubMed
-
- Deeks MJ, Calcutt JR, Ingle EK, et al. 2012. A superfamily of actin-binding proteins at the actin-membrane nexus of higher plants. Current Biology 22, 1595–1600. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

