Ageing adversely affects the migration and function of marginal zone B cells
- PMID: 28369800
- PMCID: PMC5461100
- DOI: 10.1111/imm.12737
Ageing adversely affects the migration and function of marginal zone B cells
Abstract
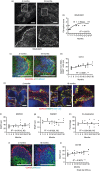
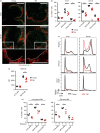
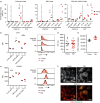
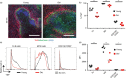
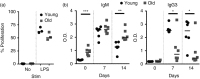
Marginal zone (MZ) B cells are positioned within the spleen to capture blood-borne antigen and immune complexes and deliver them to follicular dendritic cells in the B-cell follicles. We show that within the spleens of aged mice antigen capture by MZ B cells, and their ability to shuttle between the follicle and MZ, were impaired. The ability of aged MZ B cells to migrate towards the MZ chemoattractant sphingosine-1-phosphate was increased, suggesting that aged MZ B cells had a greater propensity to be retained within the MZ. An extrinsic impairment in aged B-cell migration towards the MZ was demonstrated using bone marrow chimeras. The follicular shuttling of MZ B cells derived from either young or aged bone marrow was similarly reduced in aged recipient spleens, showing that ageing effects on splenic stromal cells were responsible for the impaired follicular shuttling of MZ B cells. MZ B cells rapidly mount T-cell-independent (TI) antibody-responses to microbial polysaccharide antigen. In aged mice the ability to produce immunoglobulins in response to the TI type 1 antigen TNP-LPS was impaired. These ageing-related changes to the MZ and MZ B cells have implications for the clearance of blood-borne pathogens. Indeed elderly people have increased susceptibility to Streptococcus pneumoniae, a TI antigen, and decreased responses to vaccination. A thorough analysis of the mechanisms that underpin the ageing-related decline in the status of the MZ and MZ B cells will help the design of novel treatments to improve immunity in the elderly.
Keywords: B cells; T-independent responses; ageing; marginal zone; spleen.
© 2017 The Authors. Immunology Published by John Wiley & Sons Ltd.
Figures


References
-
- Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol 2005; 23:161–96. - PubMed
-
- Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T et al Sphingosine 1‐phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol 2004; 5:713–20. - PubMed
-
- Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM‐containing immune complexes onto follicular dendritic cells. Int Immunol 2004; 16:1411–22. - PubMed
-
- Fagarasan S, Honjo T. T‐Independent immune response: new aspects of B cell biology. Science 2000; 290:89–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

