A calcium-dependent pathway underlies activity-dependent plasticity of electrical synapses in the thalamic reticular nucleus
- PMID: 28369952
- PMCID: PMC5491877
- DOI: 10.1113/JP274049
A calcium-dependent pathway underlies activity-dependent plasticity of electrical synapses in the thalamic reticular nucleus
Abstract
Key points: Electrical synapses are modified by various forms of activity, including paired activity in coupled neurons and tetanization of the input to coupled neurons. We show that plasticity of electrical synapses that results from paired spiking activity in coupled neurons depends on calcium influx and calcium-initiated signalling pathways. Plasticity that results from tetanization of input fibres does not depend on calcium influx or dynamics. These results imply that electrically coupled neurons have distinct sets of mechanisms for adjusting coupling according to the specific type of activity they experience.
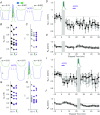
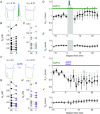
Abstract: Recent results have demonstrated modification of electrical synapse strength by varied forms of neuronal activity. However, the mechanisms underlying plasticity induction in central mammalian neurons are unclear. Here we show that the two established inductors of plasticity at electrical synapses in the thalamic reticular nucleus - paired burst spiking in coupled neurons, and mGluR-dependent tetanization of synaptic input - are separate pathways that converge at a common downstream endpoint. Using occlusion experiments and pharmacology in patched pairs of coupled neurons in vitro, we show that burst-induced depression depends on calcium entry via voltage-gated channels, is blocked by BAPTA chelation, and recruits intracellular calcium release on its way to activation of phosphatase activity. In contrast, mGluR-dependent plasticity is independent of calcium entry or calcium dynamics. Together, these results show that the spiking-initiated mechanisms underlying electrical synapse plasticity are similar to those that induce plasticity at chemical synapses, and offer the possibility that calcium-regulated mechanisms may also lead to alternate outcomes, such as potentiation. Because these mechanistic elements are widely found in mature neurons, we expect them to apply broadly to electrical synapses across the brain, acting as the crucial link between neuronal activity and electrical synapse strength.
Keywords: burst firing; calcium; depression; electrical synapse; plasticity.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures






Comment in
-
How do electrical synapses regulate their strength?J Physiol. 2017 Jul 1;595(13):4121-4122. doi: 10.1113/JP274316. Epub 2017 May 14. J Physiol. 2017. PMID: 28386941 Free PMC article. No abstract available.
References
-
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Hoher T, Matsubara M, Willecke K, Spray DC & Dermietzel R (2008). The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci USA 105, 20964–20969. - PMC - PubMed
-
- Beierlein M, Gibson JR & Connors BW (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90, 2987–3000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

