Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies
- PMID: 28370090
- PMCID: PMC5434950
- DOI: 10.1111/apt.14052
Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies
Abstract
Background: The goal of hepatorenal syndrome type 1 (HRS-1) treatment is to improve renal function. Terlipressin, a synthetic vasopressin analogue, is a systemic vasoconstrictor used for the treatment of HRS-1, where it is available.
Aim: To compare the efficacy of terlipressin plus albumin vs. placebo plus albumin in patients with HRS-1.
Methods: Pooled patient-level data from two large phase 3, randomised, placebo-controlled studies were analysed for HRS reversal [serum creatinine (SCr) value ≤133 μmol/L], 90-day survival, need for renal replacement therapy and predictors of HRS reversal. Patients received intravenous terlipressin 1-2 mg every 6 hours plus albumin or placebo plus albumin up to 14 days.
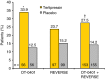
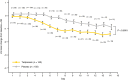
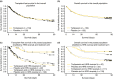
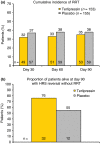
Results: The pooled analysis comprised 308 patients (terlipressin: n = 153; placebo: n = 155). HRS reversal was significantly more frequent with terlipressin vs. placebo (27% vs. 14%; P = 0.004). Terlipressin was associated with a more significant improvement in renal function from baseline until end of treatment, with a mean between-group difference in SCr concentration of -53.0 μmol/L (P < 0.0001). Lower SCr, lower mean arterial pressure and lower total bilirubin and absence of known precipitating factors for HRS were independent predictors of HRS reversal and longer survival in terlipressin-treated patients.
Conclusions: Terlipressin plus albumin resulted in a significantly higher rate of HRS reversal vs. albumin alone in patients with HRS-1. Terlipressin treatment is associated with improved renal function. (ClinicalTrials.gov identifier: OT-0401, NCT00089570; REVERSE, NCT01143246).
© 2017 The Authors. Alimentary Pharmacology and Therapeutics published by John Wiley & Sons Ltd.
Figures

Comment in
-
Editorial: tackling hepatorenal syndrome-terlipressin for all, or time for a stratified approach?Aliment Pharmacol Ther. 2017 Jul;46(2):193-194. doi: 10.1111/apt.14098. Aliment Pharmacol Ther. 2017. PMID: 28621082 No abstract available.
References
-
- Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol 2012; 9: 382–91. - PubMed
-
- Fagundes C, Gines P. Hepatorenal syndrome: a severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis 2012; 59: 874–85. - PubMed
-
- Alessandria C, Ozdogan O, Guevara M, et al MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology 2005; 41: 1282–9. - PubMed
-
- Wong F, Leung W, Al BM, Marquez M, Renner EL. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl 2015; 21: 300–7. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
