Risk-based management of invading plant disease
- PMID: 28370154
- PMCID: PMC5413851
- DOI: 10.1111/nph.14488
Risk-based management of invading plant disease
Abstract
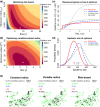
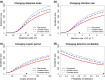
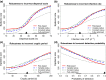
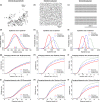
Effective control of plant disease remains a key challenge. Eradication attempts often involve removal of host plants within a certain radius of detection, targeting asymptomatic infection. Here we develop and test potentially more effective, epidemiologically motivated, control strategies, using a mathematical model previously fitted to the spread of citrus canker in Florida. We test risk-based control, which preferentially removes hosts expected to cause a high number of infections in the remaining host population. Removals then depend on past patterns of pathogen spread and host removal, which might be nontransparent to affected stakeholders. This motivates a variable radius strategy, which approximates risk-based control via removal radii that vary by location, but which are fixed in advance of any epidemic. Risk-based control outperforms variable radius control, which in turn outperforms constant radius removal. This result is robust to changes in disease spread parameters and initial patterns of susceptible host plants. However, efficiency degrades if epidemiological parameters are incorrectly characterised. Risk-based control including additional epidemiology can be used to improve disease management, but it requires good prior knowledge for optimal performance. This focuses attention on gaining maximal information from past epidemics, on understanding model transferability between locations and on adaptive management strategies that change over time.
Keywords: adaptive control; citrus canker; disease management; eradication; risk-based control; stakeholders; stochastic epidemic model.
© 2017 The Authors. New Phytologist © 2017 New Phytologist Trust.
Figures

Comment in
-
When prevention fails. Towards more efficient strategies for plant disease eradication.New Phytol. 2017 May;214(3):905-908. doi: 10.1111/nph.14555. New Phytol. 2017. PMID: 28397360 No abstract available.
References
-
- Belasque J, Bassanezi RB, Yamamoto PT, Ayres AJ, Tachibana A, Violante AR, Tank A, Di Giorgi F, Tersi FEA, Menezes GM et al 2010. Lessons from huanglongbing management in Sao Paulo state, Brazil. Journal of Plant Pathology 92: 285–302.
-
- Boyd IL, Freer‐Smith PH, Gilligan CA, Godfray HCJ. 2013. The consequence of tree pests and diseases for ecosystem services. Science 342: 1235773. - PubMed
-
- Brasier CM. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57: 792–808.
-
- Brasier CM, Webber J. 2010. Sudden larch death. Nature 466: 824–825. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

