Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: A triple-blind, randomized trial
- PMID: 28370266
- PMCID: PMC5484635
- DOI: 10.1002/ajh.24742
Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: A triple-blind, randomized trial
Abstract
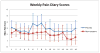
Clinical and preclinical data demonstrate that altered pulmonary physiology (including increased inflammation, increased blood flow, airway resistance, and hyper-reactivity) is an intrinsic component of Sickle Cell Disease (SCD) and may contribute to excess SCD morbidity and mortality. Inhaled corticosteroids (ICS), a safe and effective therapy for pulmonary inflammation in asthma, may ameliorate the altered pulmonary physiologic milieu in SCD. With this single-center, longitudinal, randomized, triple-blind, placebo controlled trial we studied the efficacy and feasibility of ICS in 54 nonasthmatic individuals with SCD. Participants received once daily mometasone furoate 220 mcg dry powder inhalation or placebo for 16 weeks. The primary outcome was feasibility (the number who complete the trial divided by the total number enrolled) with prespecified efficacy outcomes including daily pain score over time (patient reported) and change in soluble vascular cell adhesion molecule (sVCAM) levels between entry and 8-weeks. For the primary outcome of feasibility, the result was 96% (52 of 54, 95% CI 87%-99%) for the intent-to-treat analysis and 83% (45 of 54, 95% CI 71%-91%) for the per-protocol analysis. The adjusted treatment effect of mometasone was a reduction in daily pain score of 1.42 points (95%CI 0.61-2.21, P = 0.001). Mometasone was associated with a reduction in sVCAM levels of 526.94 ng/mL more than placebo (95% CI 50.66-1003.23, P = 0.03). These results support further study of ICS in SCD including multicenter trials and longer durations of treatment. www.clinicaltrials.gov (NCT02061202).
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease Pediatrics. 1989;84:500–8. - PubMed
-
- Petrov VP. Complications of sickle-cell anemia. Klin Med (Mosk) 1966;44:94–8. - PubMed
-
- Pianosi P, D’Souza SJ, Charge TD, Esseltine DE, Coates AL. Pulmonary function abnormalities in childhood sickle cell disease. J Pediatr. 1993;122:366–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
