Interlaboratory evaluation of a multiplexed high information content in vitro genotoxicity assay
- PMID: 28370322
- PMCID: PMC5436310
- DOI: 10.1002/em.22083
Interlaboratory evaluation of a multiplexed high information content in vitro genotoxicity assay
Abstract
We previously described a multiplexed in vitro genotoxicity assay based on flow cytometric analysis of detergent-liberated nuclei that are simultaneously stained with propidium iodide and labeled with fluorescent antibodies against p53, γH2AX, and phospho-histone H3. Inclusion of a known number of microspheres provides absolute nuclei counts. The work described herein was undertaken to evaluate the interlaboratory transferability of this assay, commercially known as MultiFlow® DNA Damage Kit-p53, γH2AX, Phospho-Histone H3. For these experiments, seven laboratories studied reference chemicals from a group of 84 representing clastogens, aneugens, and nongenotoxicants. TK6 cells were exposed to chemicals in 96-well plates over a range of concentrations for 24 hr. At 4 and 24 hr, cell aliquots were added to the MultiFlow reagent mix and following a brief incubation period flow cytometric analysis occurred, in most cases directly from a 96-well plate via a robotic walk-away data acquisition system. Multiplexed response data were evaluated using two analysis approaches, one based on global evaluation factors (i.e., cutoff values derived from all interlaboratory data), and a second based on multinomial logistic regression that considers multiple biomarkers simultaneously. Both data analysis strategies were devised to categorize chemicals as predominately exhibiting a clastogenic, aneugenic, or nongenotoxic mode of action (MoA). Based on the aggregate 231 experiments that were performed, assay sensitivity, specificity, and concordance in relation to a priori MoA grouping were ≥ 92%. These results are encouraging as they suggest that two distinct data analysis strategies can rapidly and reliably predict new chemicals' predominant genotoxic MoA based on data from an efficient and transferable multiplexed in vitro assay. Environ. Mol. Mutagen. 58:146-161, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: DNA damage; H2AX; mode of action; p53; phospho-histone H3.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
SMB, DB, JCB, and SDD are employed by Litron Laboratories. Litron has a patent covering the flow cytometry-based assay described in this manuscript and sells a commercial kit based on these procedures: MultiFlow™ DNA Damage Kit—p53, γH2AX, Phospho-Histone H3. DK serves as a consultant to Litron Laboratories.
Figures






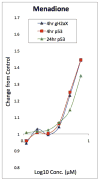
References
-
- Attia SM, Aleisa AM, Bakheet SA, Al-Yahya AA, Al-Rejaie SS, Ashour AE, Al-Shabanah OA. Molecular cytogenetic evaluation of the mechanism of micronucleus formation induced by camptothecin, topotecan, and irinotecan. Environ Mol Mutagen. 2009;50:145–151. - PubMed
-
- Attia SM. Molecular cytogenetic evaluation of the mechanism of genotoxic potential of amsacrine and nocodazole in mouse bone marrow cells. J Appl Toxicol. 2013;33:426–433. - PubMed
-
- Audebert M, Riu A, Jacques C, Hillenweck A, Jamin EL, Zalko D, Cravedi JP. Use of the γH2AX assay for assessing the genotoxicity of polycyclic aromatic hydrocarbons in human cell lines. Toxicol Lett. 2010;199:182–192. - PubMed
-
- Avlasevich SL, Bryce SM, Cairns SE, Dertinger SD. In vitro micronucleus scoring by flow cytometry: differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability. Environ Mol Mutagen. 2006;47:56–66. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

