Hydroxylated Fluorescent Dyes for Live-Cell Labeling: Synthesis, Spectra and Super-Resolution STED
- PMID: 28370443
- PMCID: PMC5599963
- DOI: 10.1002/chem.201701216
Hydroxylated Fluorescent Dyes for Live-Cell Labeling: Synthesis, Spectra and Super-Resolution STED
Abstract
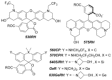
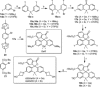
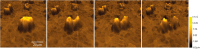
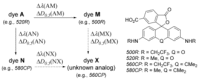
Hydroxylated rhodamines, carbopyronines, silico- and germanorhodamines with absorption maxima in the range of 530-640 nm were prepared and applied in specific labeling of living cells. The direct and high-yielding entry to germa- and silaxanthones tolerates the presence of protected heteroatoms and may be considered for the syntheses of various sila- and germafluoresceins, as well as -rhodols. Application in stimulated emission depletion (STED) fluorescence microscopy revealed a resolution of 50-75 nm in one- and two-color imaging of vimentin-HaloTag fused protein and native tubulin. The established structure-property relationships allow for prediction of the spectral properties and the positions of spirolactone/zwitterion equilibria for the new analogues of rhodamines, carbo-, silico-, and germanorhodamines using simple additive schemes.
Keywords: dyes/pigments; fluorescence; living cells; optical microscopy; rhodamines.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



References
-
- None
LinkOut - more resources
Full Text Sources
Other Literature Sources

