The Icatibant Outcome Survey: experience of hereditary angioedema management from six European countries
- PMID: 28370444
- PMCID: PMC5575527
- DOI: 10.1111/jdv.14251
The Icatibant Outcome Survey: experience of hereditary angioedema management from six European countries
Abstract
Background: Hereditary angioedema (HAE) due to C1-inhibitor deficiency (C1-INH-HAE) is a rare, potentially fatal, bradykinin-mediated disease. Icatibant is a bradykinin B2 receptor antagonist originally approved in 2008 in the European Union and 2011 in the United States as an acute therapy option for HAE attacks in adults.
Objective: To compare demographics, disease characteristics and treatment outcomes of icatibant-treated HAE attacks in patients with C1-INH-HAE enrolled in the Icatibant Outcome Survey across six European countries: Austria, France, Germany, Italy, Spain and the UK.
Methods: The Icatibant Outcome Survey [IOS; Shire, Zug, Switzerland (NCT01034969)] is an international observational study monitoring the safety and effectiveness of icatibant. Descriptive, retrospective analyses compared IOS country data derived during July 2009-April 2015.
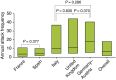
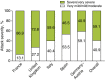
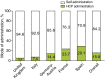
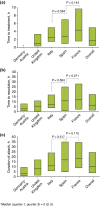
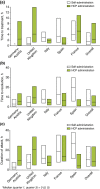
Results: Overall, 481 patients with C1-INH-HAE provided demographic data. A significant difference across countries in age at onset (P = 0.003) and baseline attack frequency (P < 0.001) was found although no significant differences were found with respect to gender (majority female; P = 0.109), age at diagnosis (P = 0.182) or delay in diagnosis (P = 0.059). Icatibant was used to treat 1893 attacks in 325 patients with majority self-administration in all countries. Overall, significant differences (all P < 0.001) were found across countries in time to treatment [median 1.8 h; median range: 0.0 (Germany-Austria) to 4.4 (France) h], time to resolution [median 6.5 h; median range: 3 (Germany-Austria) to 12 (France) h] and attack duration [median 10.5 h; median range: 3.1 (Germany-Austria) to 18.5 (France) h].
Conclusion: These data form the first European cross-country comparison of disease characteristics and icatibant use in patients with C1-INH-HAE who are enrolled in IOS. International variation in icatibant practice and treatment outcomes across the six European countries assessed highlight the need to further investigate the range of country-specific parameters driving regional variations in icatibant use.
© 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Longhurst H, Cicardi M. Hereditary angioedema. Lancet 2012; 379: 474–481. - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol 2012; 130: 692–697. - PubMed
-
- Lumry WR, Li HH, Levy RJ et al Randomized placebo‐controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST‐3 trial. Ann Allergy Asthma Immunol 2011; 107: 529–537. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

