Di-Zinc-Aryl Complexes: CO2 Insertions and Applications in Polymerisation Catalysis
- PMID: 28370511
- PMCID: PMC5488170
- DOI: 10.1002/chem.201701013
Di-Zinc-Aryl Complexes: CO2 Insertions and Applications in Polymerisation Catalysis
Abstract
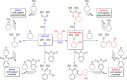
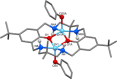
Two new di-zinc-aryl complexes, [LZn2 Ph2 ] and [LZn2 (C6 F5 )2 ], coordinated by a diphenol tetraamine macrocyclic ligand have been prepared and fully characterised, including by single-crystal X-ray diffraction experiments. The complexes' reactivities with monomers including carbon dioxide, cyclohexene oxide, phthalic anhydride, isopropanol and phenol were investigated using both experimental studies and density functional theory calculations. In particular, [LZn2 Ph2 ] readily inserts carbon dioxide to form a carboxylate, at 1 bar pressure, whereas [LZn2 (C6 F5 )2 ] does not react. Under these conditions [LZn2 Ph2 ] shows moderate activity in the ring-opening copolymerisation of cyclohexene oxide/carbon dioxide (TOF=20 h-1 ), cyclohexene oxide/phthalic anhydride (TOF=33 h-1 ) and the ring-opening polymerisations of rac-lactide (TOF=99 h-1 ) and ϵ-caprolactone (TOF=5280 h-1 ).
Keywords: CO2 insertion; organozinc catalysts; reactivity studies; ring-opening copolymerisation; ring-opening polymerisation; zinc.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures





References
-
- None
-
- Seyferth D., Organometallics 2001, 20, 2940–2955;
-
- Frankland E., J. Chem. Soc. 1850, 2, 263–296.
-
- None
-
- King A. O., Okukado N., Negishi E.-i., J. Chem. Soc. Chem. Commun. 1977, 683–684;
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

