RARα/RXR synergism potentiates retinoid responsiveness in cutaneous T-cell lymphoma cell lines
- PMID: 28370539
- PMCID: PMC5623602
- DOI: 10.1111/exd.13348
RARα/RXR synergism potentiates retinoid responsiveness in cutaneous T-cell lymphoma cell lines
Abstract
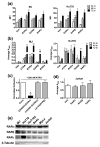
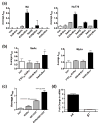
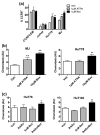
Retinoids, natural and synthetic derivatives of vitamin A, induce cellular changes by activating nuclear retinoic acid receptors (RAR) and retinoid X receptors (RXR). Although the ability of retinoids to govern gene expression is exploited clinically for cancer therapeutics, the full benefit of retinoid-based strategies is unrealized due to detrimental side effects. Delineating the receptors that prompt cellular outcomes is critical to advancing retinoid-based approaches. Here, we identify the receptors that evoke multiple responses in cutaneous T-cell lymphoma (CTCL). The data demonstrate that RARα drives integrin β7-dependent adhesion and CCR9-mediated chemotaxis in CTCL cells. Of note, concomitant activation of RARα and RXR nuclear receptors yielded synergistic increases in adhesion and migration at concentrations where single agents were ineffective. As the established paradigm of retinoid action in CTCL is apoptosis and growth arrest, the role of RARα/RXR in these events was studied. As with adhesion and migration, RARα/RXR synergism prompted apoptosis and dampened CTCL cell proliferation. Strikingly, RARα/RXR synergism induced responses from CTCL cell lines previously reported to be unresponsive to retinoids. These data provide a novel framework that may further refine a proven CTCL therapy.
Keywords: chemotaxis; integrin; lymphoma; retinoid; vitamin A.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Authors declare no conflict of interest related to this work.
Figures

References
-
- Sporn MB, Roberts AB, Goodman DS. The Retinoids Biology, Chemistry, and Medicine. New York: Ravens Press; 1994.
-
- Benbrook DM, Chambon P, Rochette-Egly C, et al. History of retinoic acid receptors. Sub-cellular biochemistry. 2014;70:1–20. - PubMed
-
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1996;10:940–954. - PubMed
-
- de Lera AR, Bourguet W, Altucci L, et al. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nature reviews Drug discovery. 2007;6:811–820. - PubMed
-
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiological reviews. 2001;81:1269–1304. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

