cpsA regulates mycotoxin production, morphogenesis and cell wall biosynthesis in the fungus Aspergillus nidulans
- PMID: 28370587
- PMCID: PMC5506848
- DOI: 10.1111/mmi.13682
cpsA regulates mycotoxin production, morphogenesis and cell wall biosynthesis in the fungus Aspergillus nidulans
Abstract
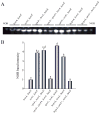
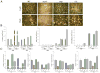
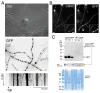
The model fungus Aspergillus nidulans synthesizes numerous secondary metabolites, including sterigmatocystin (ST). The production of this toxin is positively controlled by the global regulator veA. In the absence of veA (ΔveA), ST biosynthesis is blocked. Previously, we performed random mutagenesis in a ΔveA strain and identified revertant mutants able to synthesize ST, among them RM1. Complementation of RM1 with a genomic library revealed that the mutation occurred in a gene designated as cpsA. While in the ΔveA genetic background cpsA deletion restores ST production, in a veA wild-type background absence of cpsA reduces and delays ST biosynthesis decreasing the expression of ST genes. Furthermore, cpsA is also necessary for the production of other secondary metabolites, including penicillin, affecting the expression of PN genes. In addition, cpsA is necessary for normal asexual and sexual development. Chemical and microscopy analyses revealed that CpsA is found in cytoplasmic vesicles and it is required for normal cell wall composition and integrity, affecting adhesion capacity and oxidative stress sensitivity. The conservation of cpsA in Ascomycetes suggests that cpsA homologs might have similar roles in other fungal species.
© 2017 John Wiley & Sons Ltd.
Figures



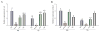




References
-
- Adams TH, Boylan MT, Timberlake WE. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988;54:353–362. - PubMed
-
- Adams TH, Yu JH. Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans. Curr Opin Microbiol. 1998;1:674–677. - PubMed
-
- Adrio JL, Demain AL. Fungal biotechnology. Int Microbiol. 2003;6:191–199. - PubMed
-
- Amare MG, Keller NP. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet Biol. 2014;66:11–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

