Aging-associated dysregulation of homeostatic immune response termination (and not initiation)
- PMID: 28371013
- PMCID: PMC5418197
- DOI: 10.1111/acel.12589
Aging-associated dysregulation of homeostatic immune response termination (and not initiation)
Abstract
Immunosenescence is a state of unbalanced immune responsiveness, characterized by a diverse repertoire of seemingly discreet and paradoxical alterations in all aspects of immunity arising in an aging-associated manner. We asked whether aging-associated alterations in the ability of apoptotic cells to elicit immunomodulatory responses (innate apoptotic immunity; IAI) or in IAI responses themselves might underlie the confounding aging-associated anomalies of immunosenescence. We explored this question by examining, as a function of animal age, responsiveness of murine macrophages on the single cell level. We monitored the expression of pro- and anti-inflammatory cytokines cytofluorimetrically in response to pro-inflammatory Toll-like receptor (TLR) stimulation and anti-inflammatory treatment with apoptotic cells. While we found no alterations with age in the potency of apoptotic cells or in the initiation and magnitude of IAI responses, we did identify a cell-intrinsic deficiency in anti-inflammatory IAI response termination linked with age and preceding manifestations of immunosenescence. Further, we found that an aging-associated deficiency in response termination also is evident following TLR stimulation. These surprising observations reveal that a loss of homeostatic immune control with animal age results from the dysregulation of response termination (as distinct from response initiation) and is exerted on the level of transcription. We suggest that, with advancing age, cells become locked into relatively longer-lived response states. Aging-associated immune dysfunctions may reflect a diminution in the cellular nimbleness of immune responsiveness.
Keywords: aging; apoptosis; homeostasis; immunosenescence; innate immunity; macrophages.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

 ) and mice of 24–25 months of age (‘old’,
) and mice of 24–25 months of age (‘old’,  ) was determined. Elicited peritoneal macrophages from individual mice within each age cohort were cultured without or with apoptotic targets (splenocytes prepared from ‘young’ syngeneic mice, treated with staurosporine) at the indicated target: responder (T: R) ratio for 2 h before the addition of
) was determined. Elicited peritoneal macrophages from individual mice within each age cohort were cultured without or with apoptotic targets (splenocytes prepared from ‘young’ syngeneic mice, treated with staurosporine) at the indicated target: responder (T: R) ratio for 2 h before the addition of  ) are calculated from the mean fluorescence intensity (
) are calculated from the mean fluorescence intensity ( ) are expressed as the fraction of all F4/80+ cells that were
) are expressed as the fraction of all F4/80+ cells that were  ), ‘middle‐aged’ (
), ‘middle‐aged’ ( ), and ‘old’ (
), and ‘old’ ( ) C57
) C57
 ) mice was determined. Elicited peritoneal macrophages from individual mice within each age cohort were cultured as indicated with apoptotic targets (as in Fig. 1A) at the indicated T: R ratio and/or
) mice was determined. Elicited peritoneal macrophages from individual mice within each age cohort were cultured as indicated with apoptotic targets (as in Fig. 1A) at the indicated T: R ratio and/or  ), ‘middle‐aged’ (
), ‘middle‐aged’ ( ) and ‘old’ (
) and ‘old’ ( ) C57
) C57
 ; A) and ‘old’ (
; A) and ‘old’ ( ; B) mice, as in Fig. 1A. Apoptotic induction of
; B) mice, as in Fig. 1A. Apoptotic induction of  ; C) and ‘old’ (
; C) and ‘old’ ( ; D) mice was determined as in Fig. 2A. Elicited peritoneal macrophages from individual mice of each age cohort were cultured with apoptotic targets (splenocytes prepared from ‘young’ [
; D) mice was determined as in Fig. 2A. Elicited peritoneal macrophages from individual mice of each age cohort were cultured with apoptotic targets (splenocytes prepared from ‘young’ [ ] or ‘old’ [
] or ‘old’ [ ] syngeneic mice, and treated with staurosporine) at the indicated T: R ratio. (Target cells were not removed.) The data presented are compiled from the results of the analysis of macrophages from 4 individual mice within each age cohort. The significance of differences between age groups, as calculated by Student's t‐test, is indicated (
] syngeneic mice, and treated with staurosporine) at the indicated T: R ratio. (Target cells were not removed.) The data presented are compiled from the results of the analysis of macrophages from 4 individual mice within each age cohort. The significance of differences between age groups, as calculated by Student's t‐test, is indicated (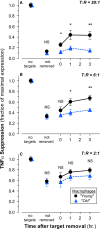
 ) C57
) C57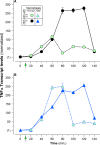
 ) and ‘old’ (B;
) and ‘old’ (B;  ) C57
) C57 ), or with
), or with  ,
, References
-
- Ahluwalia N, Mastro AM, Ball R, Miles MP, Rajendra R, Handte G (2001) Cytokine production by stimulated mononuclear cells did not change with aging in apparently healthy, well‐nourished women. Mech. Ageing Dev. 122, 1269–1279. - PubMed
-
- Birge RB, Ucker DS (2008) Innate apoptotic immunity: the calming touch of death. Cell Death Differ. 15, 1096–1102. - PubMed
-
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ (2005) Aging negatively skews macrophage TLR2‐ and TLR4‐mediated pro‐inflammatory responses without affecting the IL‐2‐stimulated pathway. Mech. Ageing Dev. 126, 1305–1313. - PubMed
-
- Bondada S, Wu H‐J, Robertson DA, Chelvarajan RL (2000) Accessory cell defect in unresponsiveness of neonates and aged to polysaccharide vaccines. Vaccine 19, 557–565. - PubMed
-
- Bruunsgaard H, Andersen‐Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK (1999) A high plasma concentration of TNF‐α is associated with dementia in centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 54, M357–M364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

