Complementary proteomic approaches reveal mitochondrial dysfunction, immune and inflammatory dysregulation in a mouse model of Gulf War Illness
- PMID: 28371386
- PMCID: PMC5637931
- DOI: 10.1002/prca.201600190
Complementary proteomic approaches reveal mitochondrial dysfunction, immune and inflammatory dysregulation in a mouse model of Gulf War Illness
Abstract
Purpose: Long-term consequences of combined pyridostigmine bromide (PB) and permethrin (PER) exposure in C57BL6/J mice using a well-characterized mouse model of exposure to these Gulf War (GW) agents were explored at the protein level.
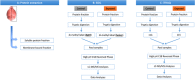
Experimental design: We used orthogonal proteomic approaches to identify pathways that are chronically impacted in the mouse CNS due to semiacute GW agent exposure early in life. These analyses were performed on soluble and membrane-bound protein fractions from brain samples using two orthogonal isotopic labeling LC-MS/MS proteomic approaches-stable isotope dimethyl labeling and iTRAQ.
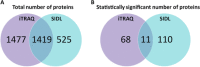
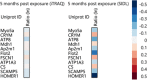
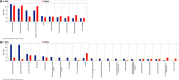
Results: The use of these approaches allowed for greater coverage of proteins than was possible by either one alone and revealed both distinct and overlapping datasets. This combined analysis identified changes in several mitochondrial, as well as immune and inflammatory pathways after GW agent exposure.
Conclusions and clinical relevance: The work discussed here provides insight into GW agent exposure dependent mechanisms that adversely affect mitochondrial function and immune and inflammatory regulation. Collectively, our work identified key pathways which were chronically impacted in the mouse CNS following acute GW agent exposure, this may lead to the identification of potential targets for therapeutic intervention in the future. Long-term consequences of combined PB and PER exposure in C57BL6/J mice using a well-characterized mouse model of exposure to these GW agents were explored at the protein level. Expanding on earlier work, we used orthogonal proteomic approaches to identify pathways that are chronically impacted in the mouse CNS due to semiacute GW agent exposure early in life. These analyses were performed on soluble and membrane-bound protein fractions from brain samples using two orthogonal isotopic labeling LC-MS/MS proteomic approaches-stable isotope dimethyl labeling and iTRAQ. The use of these approaches allowed for greater coverage of proteins than was possible by either one alone and revealed both distinct and overlapping datasets. This combined analysis identified changes in several mitochondrial, as well as immune and inflammatory pathways after GW agent exposure. The work discussed here provides insight into GW agent exposure dependent mechanisms that adversely affect mitochondrial function and immune and inflammatory regulation at 5 months postexposure to PB + PER.
Keywords: Gulf War; MS/MS; SIDL; iTRAQ; mitochondrial dysfunction.
© 2017 The Authors. PROTEOMICS - Clinical Applications published by WILEY-VCH Verlag GmbH & Co. KGaA.
Figures

References
-
- Wille, T. , Thiermann, H. , Worek, F. , In vitro kinetic interactions of DEET, pyridostigmine and organophosphorus pesticides with human cholinesterases. Chem. Biol. Interact. 2011, 190, 79–83. - PubMed
-
- Binns, J. B. C. , Bloom, E. , Clauw, D. , Gulf war illness and the health of gulf war veterans. Research Advisory Committee on Gulf War Veterans’ Illnesses 2008.
-
- Ojo, J. O. , Abdullah, L. , Evans, J. , Reed, J. M. et al., Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 2013, 34, 109–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

