Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain
- PMID: 28371821
- PMCID: PMC5570194
- DOI: 10.1093/neuonc/nox027
Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain
Abstract
Background: Ependymomas account for up to 10% of childhood CNS tumors and have a high rate of tumor recurrence despite gross total resection. Recently, classification into molecular ependymoma subgroups has been established, but the mechanisms underlying the aggressiveness of certain subtypes remain widely enigmatic. The aim of this study was to dissect the clinical and biological role of telomerase reactivation, a frequent mechanism of cancer cells to evade cellular senescence, in pediatric ependymoma.
Methods: We determined telomerase enzymatic activity, hTERT mRNA expression, promoter methylation, and the rs2853669 single nucleotide polymorphism located in the hTERT promoter in a well-characterized cohort of pediatric intracranial ependymomas.
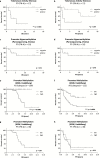
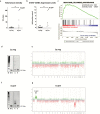
Results: In posterior fossa ependymoma group A (PF-EPN-A) tumors, telomerase activity varied and was significantly associated with dismal overall survival, whereas telomerase reactivation was present in all supratentorial RelA fusion-positive (ST-EPN-RELA) ependymomas. In silico analysis of methylation patterns showed that only these two subgroups harbor hypermethylated hTERT promoters suggesting telomerase reactivation via epigenetic mechanisms. Furthermore, chromosome 1q gain, a well-known negative prognostic factor, was strongly associated with telomerase reactivation in PF-EPN-A. Additional in silico analyses of gene expression data confirmed this finding and further showed enrichment of the E-twenty-six factor, Myc, and E2F target genes in 1q gained ependymomas. Additionally, 1q gained tumors showed elevated expression of ETV3, an E-twenty-six factor gene located on chromosome 1q.
Conclusion: Taken together we describe a subgroup-specific impact of telomerase reactivation on disease progression in pediatric ependymoma and provide preliminary evidence for the involved molecular mechanisms.
Keywords: RelA fusion; chromosome 1q; ependymoma; promoter methylation; telomerase.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures



Comment in
-
Can telomerase activity be unleashed to refine prognosis within ependymoma subgroups?Neuro Oncol. 2017 Sep 1;19(9):1149-1151. doi: 10.1093/neuonc/nox059. Neuro Oncol. 2017. PMID: 28521052 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

