Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial
- PMID: 28372335
- PMCID: PMC5409050
- DOI: 10.3233/JAD-161256
Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial
Abstract
Background: Long acting insulin detemir administered intranasally for three weeks enhanced memory for adults with Alzheimer's disease dementia (AD) or amnestic mild cognitive impairment (MCI). The investigation of longer-term administration is necessary to determine whether benefits persist, whether they are similar to benefits provided by regular insulin, and whether either form of insulin therapy affects AD biomarkers.
Objective: The present study aimed to determine whether four months of treatment with intranasal insulin detemir or regular insulin improves cognition, daily functioning, and AD biomarkers for adults with MCI or AD.
Methods: This randomized, double-blind, placebo-controlled trial included an intent-to-treat sample consisting of 36 adults diagnosed with MCI or mild to moderate AD. Participants received placebo (n = 12), 40 IU of insulin detemir (n = 12), or 40 IU of regular insulin (n = 12) daily for four months, administered with a nasal delivery device. A cognitive battery was administered at baseline and after two and four months of treatment. MRI was administered for all participants and lumbar puncture for a subset (n = 20) at baseline and four months. The primary outcome was change from baseline to four months on a memory composite (sum of Z scores for delayed list and story recall). Secondary outcomes included: global cognition (Alzheimer's Disease Assessment Scale-Cognition), daily functioning (Dementia Severity Rating Scale), MRI volume changes in AD-related regions of interest, and cerebrospinal fluid AD markers.
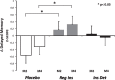
Results: The regular insulin treated group had better memory after two and four months compared with placebo (p < 0.03). No significant effects were observed for the detemir-assigned group compared with the placebo group, or for daily functioning for either group. Regular insulin treatment was associated with preserved volume on MRI. Regular insulin treatment was also associated with reduction in the tau-P181/Aβ42 ratio.
Conclusion: Future research is warranted to examine the mechanistic basis of treatment differences, and to further assess the efficacy and safety of intranasal insulin.
Keywords: Alzheimer’s disease; clinical trial; insulin; intranasal; magnetic resonance imaging; memory.
Figures


References
-
- Ribe EM, Lovestone S (2016) Insulin signalling in Alzheimer’s disease and diabetes: From epidemiology to molecular links. J Intern Med 280, 430–442. - PubMed
-
- Lee CC, Kuo YM, Huang CC, Hsu KS (2009) Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging 30, 377–387. - PubMed
-
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL (2009) Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A 106, 1971–1976. - PMC - PubMed
-
- Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY, Louneva N, Lee VM, Kim SF, Trojanowski JQ, Arnold SE (2014) Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol 128, 679–689. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

