A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-γ and IFN-λ) and Type 2 Inflammation (IL-5 and IL-13)
- PMID: 28373098
- PMCID: PMC5440599
- DOI: 10.1016/j.ebiom.2017.03.033
A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-γ and IFN-λ) and Type 2 Inflammation (IL-5 and IL-13)
Abstract
Background: Rhinovirus infection is a major cause of asthma exacerbations.
Objectives: We studied nasal and bronchial mucosal inflammatory responses during experimental rhinovirus-induced asthma exacerbations.
Methods: We used nasosorption on days 0, 2-5 and 7 and bronchosorption at baseline and day 4 to sample mucosal lining fluid to investigate airway mucosal responses to rhinovirus infection in patients with allergic asthma (n=28) and healthy non-atopic controls (n=11), by using a synthetic absorptive matrix and measuring levels of 34 cytokines and chemokines using a sensitive multiplex assay.
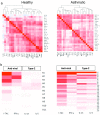
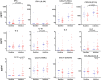
Results: Following rhinovirus infection asthmatics developed more upper and lower respiratory symptoms and lower peak expiratory flows compared to controls (all P<0.05). Asthmatics also developed higher nasal lining fluid levels of an anti-viral pathway (including IFN-γ, IFN-λ/IL-29, CXCL11/ITAC, CXCL10/IP10 and IL-15) and a type 2 inflammatory pathway (IL-4, IL-5, IL-13, CCL17/TARC, CCL11/eotaxin, CCL26/eotaxin-3) (area under curve day 0-7, all P<0.05). Nasal IL-5 and IL-13 were higher in asthmatics at day 0 (P<0.01) and levels increased by days 3 and 4 (P<0.01). A hierarchical correlation matrix of 24 nasal lining fluid cytokine and chemokine levels over 7days demonstrated expression of distinct interferon-related and type 2 pathways in asthmatics. In asthmatics IFN-γ, CXCL10/IP10, CXCL11/ITAC, IL-15 and IL-5 increased in bronchial lining fluid following viral infection (all P<0.05).
Conclusions: Precision sampling of mucosal lining fluid identifies robust interferon and type 2 responses in the upper and lower airways of asthmatics during an asthma exacerbation. Nasosorption and bronchosorption have potential to define asthma endotypes in stable disease and at exacerbation.
Keywords: Absorption of mucosal lining fluid; Asthma; Interferons; Mucosal immunology; Rhinovirus; Type II inflammation.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





Comment in
-
Mucosal Lining Fluid Biomarkers in Asthma: Basis for Rational Use of New Targeted Therapies?EBioMedicine. 2017 May;19:12-13. doi: 10.1016/j.ebiom.2017.04.016. Epub 2017 Apr 11. EBioMedicine. 2017. PMID: 28412250 Free PMC article. No abstract available.
References
-
- Alam R., Sim T.C., Hilsmeier K., Grant J.A. Development of a new technique for recovery of cytokines from inflammatory sites in situ. J. Immunol. Methods. 1992;155:25–29. - PubMed
-
- Bardin P.G., Sanderson G., Robinson B.S., Holgate S.T., Tyrrell D.A. Experimental rhinovirus infection in volunteers. Eur. Respir. J. 1996;9:2250–2255. - PubMed
-
- Beale J., Jayaraman A., Jackson D.J., Macintyre J.D., Edwards M.R., Walton R.P., Zhu J., Ching Y.M., Shamji B., Edwards M., Westwick J., Cousins D.J., Hwang Y.Y., McKenzie A., Johnston S.L., Bartlett N.W. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014;6 (256ra134) - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

