Prospective Evaluation of PI-RADS™ Version 2 Using the International Society of Urological Pathology Prostate Cancer Grade Group System
- PMID: 28373133
- PMCID: PMC7900896
- DOI: 10.1016/j.juro.2017.03.131
Prospective Evaluation of PI-RADS™ Version 2 Using the International Society of Urological Pathology Prostate Cancer Grade Group System
Abstract
Purpose: The PI-RADS™ (Prostate Imaging Reporting and Data System), version 2 scoring system, introduced in 2015, is based on expert consensus. In the same time frame ISUP (International Society of Urological Pathology) introduced a new pathological scoring system for prostate cancer. Our goal was to prospectively evaluate the cancer detection rates for each PI-RADS, version 2 category and compare them to ISUP group scores in patients undergoing systematic biopsy and magnetic resonance imaging-transrectal ultrasound fusion guided biopsy.
Materials and methods: A total of 339 treatment naïve patients prospectively underwent multiparametric magnetic resonance imaging evaluated with PI-RADS, version 2 with subsequent systematic and fusion guided biopsy from May 2015 to May 2016. ISUP scores were applied to pathological specimens. An ISUP score of 2 or greater (ie Gleason 3 + 4 or greater) was defined as clinically significant prostate cancer. Cancer detection rates were determined for each PI-RADS, version 2 category as well as for the T2 weighted PI-RADS, version 2 categories in the peripheral zone.
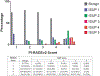
Results: The cancer detection rate for PI-RADS, version 2 categories 1, 2, 3, 4 and 5 was 25%, 20.2%, 24.8%, 39.1% and 86.9% for all prostate cancer, and 0%, 9.6%, 12%, 22.1% and 72.4% for clinically significant prostate cancer, respectively. On T2-weighted magnetic resonance imaging the cancer detection rate in the peripheral zone was significantly higher for PI-RADS, version 2 category 4 than for overall PI-RADS, version 2 category 4 in the peripheral zone (all prostate cancer 36.6% vs 48.1%, p = 0.001, and clinically significant prostate cancer 22.9% vs 32.6%, p = 0.002).
Conclusions: The cancer detection rate increases with higher PI-RADS, version 2 categories.
Keywords: biopsy; diagnostic imaging; early detection of cancer; prostatic neoplasms; reference standards.
Copyright © 2017 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Editorial Comment.J Urol. 2017 Sep;198(3):590. doi: 10.1016/j.juro.2017.03.149. Epub 2017 Jun 13. J Urol. 2017. PMID: 28627480 No abstract available.
-
Intermediate Risk Prostate Cancer and Active Surveillance: Maximize Utilization while Minimizing Failure.J Urol. 2017 Sep;198(3):493-495. doi: 10.1016/j.juro.2017.06.055. Epub 2017 Jun 17. J Urol. 2017. PMID: 28628758 No abstract available.
References
-
- Miller KD, Siegel RL, Lin CC et al.: Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J et al.: EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2013; 65: 124. - PubMed
-
- Jones JS: Saturation biopsy for detecting and characterizing prostate cancer. BJU Int 2007; 99: 1340. - PubMed
-
- Lane BR, Zippe CD, Abouassaly R et al.: Saturation technique does not decrease cancer detection during followup after initial prostate biopsy. J Urol 2008; 179: 1746. - PubMed
-
- Jones JS, Patel A, Schoenfield L et al.: Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175: 485. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

