Lung Tissue Concentrations of Pyrazinamide among Patients with Drug-Resistant Pulmonary Tuberculosis
- PMID: 28373198
- PMCID: PMC5444116
- DOI: 10.1128/AAC.00226-17
Lung Tissue Concentrations of Pyrazinamide among Patients with Drug-Resistant Pulmonary Tuberculosis
Abstract
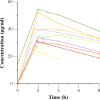
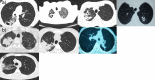
Improved knowledge regarding the tissue penetration of antituberculosis drugs may help optimize drug management. Patients with drug-resistant pulmonary tuberculosis undergoing adjunctive surgery were enrolled. Serial serum samples were collected, and microdialysis was performed using ex vivo lung tissue to measure pyrazinamide concentrations. Among 10 patients, the median pyrazinamide dose was 24.7 mg/kg of body weight. Imaging revealed predominant lung lesions as cavitary (n = 6 patients), mass-like (n = 3 patients), or consolidative (n = 1 patient). On histopathology examination, all tissue samples had necrosis; eight had a pH of ≤5.5. Tissue samples from two patients were positive for Mycobacterium tuberculosis by culture (pH 5.5 and 7.2). All 10 patients had maximal serum pyrazinamide concentrations within the recommended range of 20 to 60 μg/ml. The median lung tissue free pyrazinamide concentration was 20.96 μg/ml. The median tissue-to-serum pyrazinamide concentration ratio was 0.77 (range, 0.54 to 0.93). There was a significant inverse correlation between tissue pyrazinamide concentrations and the amounts of necrosis (R = -0.66, P = 0.04) and acid-fast bacilli (R = -0.75, P = 0.01) identified by histopathology. We found good penetration of pyrazinamide into lung tissue among patients with pulmonary tuberculosis with a variety of radiological lesion types. Our tissue pH results revealed that most lesions had a pH conducive to pyrazinamide activity. The tissue penetration of pyrazinamide highlights its importance in both drug-susceptible and drug-resistant antituberculosis treatment regimens.
Keywords: drug penetration; drug resistance; microdialysis; pharmacology; pyrazinamide; tuberculosis.
Copyright © 2017 American Society for Microbiology.
Figures


Comment in
-
pH Conditions under Which Pyrazinamide Works in Humans.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00854-17. doi: 10.1128/AAC.00854-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28839079 Free PMC article. No abstract available.
-
Reply to Srivastava et al., "pH Conditions under Which Pyrazinamide Works in Humans".Antimicrob Agents Chemother. 2017 Aug 24;61(9):e01120-17. doi: 10.1128/AAC.01120-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28839080 Free PMC article. No abstract available.
References
-
- World Health Organization. 2016. Global tuberculosis report 2016. Report WHO/HTM/TB/2016.13. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2015. Implementing the end TB strategy: the essentials. WHO/HTM/TB/2015.31 World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

