Efficacy, Safety, and Tolerability of Gepotidacin (GSK2140944) in the Treatment of Patients with Suspected or Confirmed Gram-Positive Acute Bacterial Skin and Skin Structure Infections
- PMID: 28373199
- PMCID: PMC5444153
- DOI: 10.1128/AAC.02095-16
Efficacy, Safety, and Tolerability of Gepotidacin (GSK2140944) in the Treatment of Patients with Suspected or Confirmed Gram-Positive Acute Bacterial Skin and Skin Structure Infections
Abstract
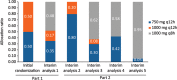
Gepotidacin is a novel, first-in-class, triazaacenaphthylene antibacterial agent which has in vitro activity against causative pathogens of acute bacterial skin and skin structure infections (ABSSSIs). This phase 2, randomized, 2-part, multicenter, dose-ranging, response-adaptive study with optional intravenous-oral switch evaluated the efficacy and safety of gepotidacin for the treatment of Gram-positive ABSSSIs in 122 adult patients in the United States. The study had a double-blind phase (part 1; intravenous [750 mg or 1,000 mg every 12 h {q12h}]) and an open-label phase (part 2; intravenous [750 mg q12h, 1,000 mg q12h, or 1,000 q8h]). The primary endpoint was a composite of efficacy and safety which consisted of the early cure rate and the withdrawal rate due to drug-related adverse events and utilized a clinical utility index for dose selection. At the early efficacy visit (48 to 72 h after the first dose), the 750-mg q12h and 1,000-mg q8h groups met prespecified success criteria for clinical utility in terms of efficacy and safety; however, the 1,000-mg q12h group did not meet these criteria due to observed lower efficacy rates. The most frequently reported adverse events were nausea (20%) and diarrhea (13%). These encouraging phase 2 results demonstrate the potential for gepotidacin to meet the medical need for novel antibacterial agents to treat ABSSSIs due to drug-resistant pathogens through a unique mechanism of action. (This study has been registered at ClinicalTrials.gov under registration no. NCT02045797.).
Keywords: ABSSSI; antibacterial agent; antimicrobial safety; efficacy; gepotidacin; phase 2 study; skin infections.
Copyright © 2017 O'Riordan et al.
Figures


References
-
- Suaya JA, Mera RM, Cassidy A, O'Hara P, Amrine-Madsen H, Burstin S, Miller LG. 2014. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 14:296. doi:10.1186/1471-2334-14-296. - DOI - PMC - PubMed
-
- Pfuntner A, Wier LM, Stocks C. 2013. Most frequent conditions in U.S. hospitals, 2011. Statistical brief 162. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Agency for Healthcare Policy and Research, Rockville, MD: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf Accessed 1 June 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

