Defining the RNaseH2 enzyme-initiated ribonucleotide excision repair pathway in Archaea
- PMID: 28373277
- PMCID: PMC5448109
- DOI: 10.1074/jbc.M117.783472
Defining the RNaseH2 enzyme-initiated ribonucleotide excision repair pathway in Archaea
Abstract
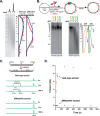
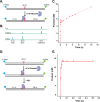
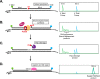
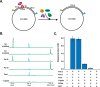
Incorporation of ribonucleotides during DNA replication has severe consequences for genome stability. Although eukaryotes possess a number of redundancies for initiating and completing repair of misincorporated ribonucleotides, archaea such as Thermococcus rely only upon RNaseH2 to initiate the pathway. Because Thermococcus DNA polymerases incorporate as many as 1,000 ribonucleotides per genome, RNaseH2 must be efficient at recognizing and nicking at embedded ribonucleotides to ensure genome integrity. Here, we show that ribonucleotides are incorporated by the hyperthermophilic archaeon Thermococcus kodakarensis both in vitro and in vivo and a robust ribonucleotide excision repair pathway is critical to keeping incorporation levels low in wild-type cells. Using pre-steady-state and steady-state kinetics experiments, we also show that archaeal RNaseH2 rapidly cleaves at embedded ribonucleotides (200-450 s-1), but exhibits an ∼1,000-fold slower turnover rate (0.06-0.17 s-1), suggesting a potential role for RNaseH2 in protecting or marking nicked sites for further processing. We found that following RNaseH2 cleavage, the combined activities of polymerase B (PolB), flap endonuclease (Fen1), and DNA ligase are required to complete ribonucleotide processing. PolB formed a ribonucleotide-containing flap by strand displacement synthesis that was cleaved by Fen1, and DNA ligase sealed the nick for complete repair. Our study reveals conservation of the overall mechanism of ribonucleotide excision repair across domains of life. The lack of redundancies in ribonucleotide repair in archaea perhaps suggests a more ancestral form of ribonucleotide excision repair compared with the eukaryotic pathway.
Keywords: DNA damage; DNA repair; DNA replication; DNA synthesis; RNaseH2; archaea; enzyme kinetics; pre-steady-state kinetics; ribonucleotide excision repair.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
The roles of family B and D DNA polymerases in Thermococcus species 9°N Okazaki fragment maturation.J Biol Chem. 2015 May 15;290(20):12514-22. doi: 10.1074/jbc.M115.638130. Epub 2015 Mar 26. J Biol Chem. 2015. PMID: 25814667 Free PMC article.
-
Pre-steady-state Kinetic Analysis of a Family D DNA Polymerase from Thermococcus sp. 9°N Reveals Mechanisms for Archaeal Genomic Replication and Maintenance.J Biol Chem. 2015 Sep 4;290(36):21800-10. doi: 10.1074/jbc.M115.662841. Epub 2015 Jul 9. J Biol Chem. 2015. PMID: 26160179 Free PMC article.
-
Biochemical reconstitution and genetic characterization of the major oxidative damage base excision DNA repair pathway in Thermococcus kodakarensis.DNA Repair (Amst). 2020 Feb;86:102767. doi: 10.1016/j.dnarep.2019.102767. Epub 2019 Dec 5. DNA Repair (Amst). 2020. PMID: 31841800 Free PMC article.
-
Redundancy in ribonucleotide excision repair: Competition, compensation, and cooperation.DNA Repair (Amst). 2015 May;29:74-82. doi: 10.1016/j.dnarep.2015.02.008. Epub 2015 Feb 16. DNA Repair (Amst). 2015. PMID: 25753809 Free PMC article. Review.
-
Molecular and physiological consequences of faulty eukaryotic ribonucleotide excision repair.EMBO J. 2020 Feb 3;39(3):e102309. doi: 10.15252/embj.2019102309. Epub 2019 Dec 12. EMBO J. 2020. PMID: 31833079 Free PMC article. Review.
Cited by
-
A study on endonuclease BspD6I and its stimulus-responsive switching by modified oligonucleotides.PLoS One. 2018 Nov 26;13(11):e0207302. doi: 10.1371/journal.pone.0207302. eCollection 2018. PLoS One. 2018. PMID: 30475809 Free PMC article.
-
Capillary Electrophoresis-Based Functional Genomics Screening to Discover Novel Archaeal DNA Modifying Enzymes.Appl Environ Microbiol. 2022 Jan 25;88(2):e0213721. doi: 10.1128/AEM.02137-21. Epub 2021 Nov 17. Appl Environ Microbiol. 2022. PMID: 34788065 Free PMC article.
-
Novel ribonucleotide discrimination in the RNA polymerase-like two-barrel catalytic core of Family D DNA polymerases.Nucleic Acids Res. 2020 Dec 2;48(21):12204-12218. doi: 10.1093/nar/gkaa986. Nucleic Acids Res. 2020. PMID: 33137176 Free PMC article.
-
Unlike the Escherichia coli counterpart, archaeal RNase HII cannot process ribose monophosphate abasic sites and oxidized ribonucleotides embedded in DNA.J Biol Chem. 2019 Aug 30;294(35):13061-13072. doi: 10.1074/jbc.RA119.009493. Epub 2019 Jul 12. J Biol Chem. 2019. PMID: 31300556 Free PMC article.
-
Ribonucleotide incorporation into DNA during DNA replication and its consequences.Crit Rev Biochem Mol Biol. 2021 Feb;56(1):109-124. doi: 10.1080/10409238.2020.1869175. Epub 2021 Jan 18. Crit Rev Biochem Mol Biol. 2021. PMID: 33461360 Free PMC article. Review.
References
-
- Traut T. W. (1994) Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

