An immunoaffinity-based method for isolating ultrapure adult astrocytes based on ATP1B2 targeting by the ACSA-2 antibody
- PMID: 28373281
- PMCID: PMC5448122
- DOI: 10.1074/jbc.M116.765313
An immunoaffinity-based method for isolating ultrapure adult astrocytes based on ATP1B2 targeting by the ACSA-2 antibody
Abstract
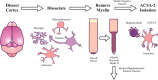
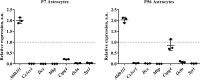
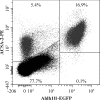
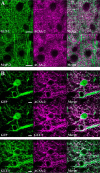
Astrocytes are a major cell type in the mammalian CNS. Astrocytes are now known to play a number of essential roles in processes including synapse formation and function, as well as blood-brain barrier formation and control of cerebral blood flow. However, our understanding of the molecular mechanisms underlying astrocyte development and function is still rudimentary. This lack of knowledge is at least partly due to the lack of tools currently available for astrocyte biology. ACSA-2 is a commercially available antibody originally developed for the isolation of astrocytes from young postnatal mouse brain, using magnetic cell-sorting methods, but its utility in isolating cells from adult tissue has not yet been published. Using a modified protocol, we now show that this tool can also be used to isolate ultrapure astrocytes from the adult brain. Furthermore, using a variety of techniques (including single-cell sequencing, overexpression and knockdown assays, immunoblotting, and immunohistochemistry), we identify the ACSA-2 epitope for the first time as ATP1B2 and characterize its distribution in the CNS. Finally, we show that ATP1B2 is stably expressed in multiple models of CNS injury and disease. Hence, we show that the ACSA-2 antibody possesses the potential to be an extremely valuable tool for astrocyte research, allowing the purification and characterization of astrocytes (potentially including injury and disease models) without the need for any specialized and expensive equipment. In fact, our results suggest that ACSA-2 should be a first-choice method for astrocyte isolation and characterization.
Keywords: astrocyte; cell surface protein; flow cytometry; immunoblotting; immunohistochemistry; immunoisolation; membrane protein; neuroscience; plasma membrane; single-cell RNA-seq.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Barres B. A. (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 - PubMed
-
- Doetsch F., Caillé I., Lim D. A., García-Verdugo J. M., and Alvarez-Buylla A. (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

