States With Prescription Drug Monitoring Mandates Saw A Reduction In Opioids Prescribed To Medicaid Enrollees
- PMID: 28373340
- PMCID: PMC5625882
- DOI: 10.1377/hlthaff.2016.1141
States With Prescription Drug Monitoring Mandates Saw A Reduction In Opioids Prescribed To Medicaid Enrollees
Abstract
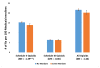
Prescription drug monitoring programs are promising tools to use in addressing the prescription opioid epidemic, yet prescribers' participation in these state-run programs remained low as of 2014. Statutory mandates for prescribers to register with their state's program, use it, or both are believed to be effective tools to realize the programs' full potential. Our analysis of aggregate Medicaid drug utilization data indicates that state mandates for prescriber registration or use adopted in 2011-14 were associated with a reduction of 9-10 percent in population-adjusted numbers of Schedule II opioid prescriptions received by Medicaid enrollees and amounts of Medicaid spending on these prescriptions. This effect was largely associated with mandates of registration, which were comprehensive in all adopting states, and not with mandates of use, which were largely limited in scope or strength before 2015. Our findings support the use of mandates of registration in prescription drug monitoring programs as an effective and relatively low-cost policy. Future research should further assess the value of strong mandates of use to ensure safer and more appropriate prescribing of opioids.
Keywords: Behavioral Health; Medicaid; Opioids; Prescription Drug Monitoring Programs.
Project HOPE—The People-to-People Health Foundation, Inc.
Figures

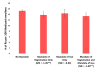

References
-
- Volkow ND. Hearing before the Senate Caucus on International Narcotics Control [Internet] Washington (DC): US Congress; 2014. May 14, [cited 2016 Aug 16]. Available to download from: https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to....
-
- RTI International. Results from the 2014 National Survey on Drug Use and Health: detailed tables [Internet] Rockville (MD): Substance Abuse and Mental Health Services Administration; 2015. Sep 10, [cited 2017 Feb 10]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-D....
-
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose–related deaths. JAMA. 2011;305(13):1315–21. - PubMed
-
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–82. (MMWR) - PubMed
-
- Department of Health and Human Services, Behavioral Health Coordinating Committee, Prescription Drug Abuse Subcommittee. Addressing prescription drug abuse in the United States: current activities and future opportunities [Internet] Washington (DC): HHS; [cited 2017 Feb 10]. Available from: http://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_0....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

