Strategies for Increasing Pancreatic Tumor Immunogenicity
- PMID: 28373364
- PMCID: PMC5466881
- DOI: 10.1158/1078-0432.CCR-16-2318
Strategies for Increasing Pancreatic Tumor Immunogenicity
Abstract
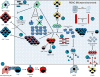
Immunotherapy has changed the standard of care for multiple deadly cancers, including lung, head and neck, gastric, and some colorectal cancers. However, single-agent immunotherapy has had little effect in pancreatic ductal adenocarcinoma (PDAC). Increasing evidence suggests that the PDAC microenvironment is comprised of an intricate network of signals between immune cells, PDAC cells, and stroma, resulting in an immunosuppressive environment resistant to single-agent immunotherapies. In this review, we discuss differences between immunotherapy-sensitive cancers and PDAC, the complex interactions between PDAC stroma and suppressive tumor-infiltrating cells that facilitate PDAC development and progression, the immunologic targets within these complex networks that are druggable, and data supporting combination drug approaches that modulate multiple PDAC signals, which should lead to improved clinical outcomes. Clin Cancer Res; 23(7); 1656-69. ©2017 AACRSee all articles in this CCR Focus section, "Pancreatic Cancer: Challenge and Inspiration."
©2017 American Association for Cancer Research.
Figures

References
-
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. Available from: - PubMed
-
- Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

