Plasmodium falciparum erythrocyte-binding antigen 175 triggers a biophysical change in the red blood cell that facilitates invasion
- PMID: 28373555
- PMCID: PMC5402469
- DOI: 10.1073/pnas.1620843114
Plasmodium falciparum erythrocyte-binding antigen 175 triggers a biophysical change in the red blood cell that facilitates invasion
Abstract
Invasion of the red blood cell (RBC) by the Plasmodium parasite defines the start of malaria disease pathogenesis. To date, experimental investigations into invasion have focused predominantly on the role of parasite adhesins or signaling pathways and the identity of binding receptors on the red cell surface. A potential role for signaling pathways within the erythrocyte, which might alter red cell biophysical properties to facilitate invasion, has largely been ignored. The parasite erythrocyte-binding antigen 175 (EBA175), a protein required for entry in most parasite strains, plays a key role by binding to glycophorin A (GPA) on the red cell surface, although the function of this binding interaction is unknown. Here, using real-time deformability cytometry and flicker spectroscopy to define biophysical properties of the erythrocyte, we show that EBA175 binding to GPA leads to an increase in the cytoskeletal tension of the red cell and a reduction in the bending modulus of the cell's membrane. We isolate the changes in the cytoskeleton and membrane and show that reduction in the bending modulus is directly correlated with parasite invasion efficiency. These data strongly imply that the malaria parasite primes the erythrocyte surface through its binding antigens, altering the biophysical nature of the target cell and thus reducing a critical energy barrier to invasion. This finding would constitute a major change in our concept of malaria parasite invasion, suggesting it is, in fact, a balance between parasite and host cell physical forces working together to facilitate entry.
Keywords: erythrocyte; flicker spectroscopy; malaria; merozoite; real-time deformability cytometry.
Conflict of interest statement
Conflict of interest statement: O.O. is a cofounder of a company commercializing real-time deformability cytometry.
Figures





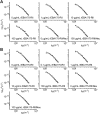

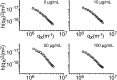


References
-
- WHO . World Malaria Report. World Health Organization (WHO); Geneva, Switzerland: 2016.
-
- Riglar DT, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011;9:9–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

