Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14
- PMID: 28373577
- PMCID: PMC5402431
- DOI: 10.1073/pnas.1616733114
Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14
Abstract
Renal Ca2+ reabsorption is essential for maintaining systemic Ca2+ homeostasis and is tightly regulated through the parathyroid hormone (PTH)/PTHrP receptor (PTH1R) signaling pathway. We investigated the role of PTH1R in the kidney by generating a mouse model with targeted deletion of PTH1R in the thick ascending limb of Henle (TAL) and in distal convoluted tubules (DCTs): Ksp-cre;Pth1rfl/fl Mutant mice exhibited hypercalciuria and had lower serum calcium and markedly increased serum PTH levels. Unexpectedly, proteins involved in transcellular Ca2+ reabsorption in DCTs were not decreased. However, claudin14 (Cldn14), an inhibitory factor of the paracellular Ca2+ transport in the TAL, was significantly increased. Analyses by flow cytometry as well as the use of Cldn14-lacZ knock-in reporter mice confirmed increased Cldn14 expression and promoter activity in the TAL of Ksp-cre;Pth1rfl/fl mice. Moreover, PTH treatment of HEK293 cells stably transfected with CLDN14-GFP, together with PTH1R, induced cytosolic translocation of CLDN14 from the tight junction. Furthermore, mice with high serum PTH levels, regardless of high or low serum calcium, demonstrated that PTH/PTH1R signaling exerts a suppressive effect on Cldn14. We therefore conclude that PTH1R signaling directly and indirectly regulates the paracellular Ca2+ transport pathway by modulating Cldn14 expression in the TAL. Finally, systemic deletion of Cldn14 completely rescued the hypercalciuric and lower serum calcium phenotype in Ksp-cre;Pth1rfl/fl mice, emphasizing the importance of PTH in inhibiting Cldn14. Consequently, suppressing CLDN14 could provide a potential treatment to correct urinary Ca2+ loss, particularly in patients with hypoparathyroidism.
Keywords: CLDN14; PTH1R; hypercalciuria; mouse kidney; paracellular.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
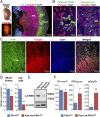

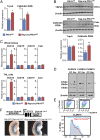







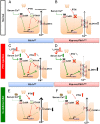
References
-
- Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5:S23–S30. - PubMed
-
- Bihl G, Meyers A. Recurrent renal stone disease-advances in pathogenesis and clinical management. Lancet. 2001;358:651–656. - PubMed
-
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: A review of the recent evidence. Endocr Pract. 2006;12:436–445. - PubMed
-
- Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19:120–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

