PEG-asparaginase in BFM-90 regimen improves outcomes in adults with newly diagnosed lymphoblastic lymphoma
- PMID: 28373755
- PMCID: PMC5348477
- DOI: 10.21147/j.issn.1000-9604.2017.01.08
PEG-asparaginase in BFM-90 regimen improves outcomes in adults with newly diagnosed lymphoblastic lymphoma
Abstract
Objective: Although L-asparaginase (L-ASP) is a standard treatment for lymphoblastic lymphoma (LBL), hypersensitivity reactions by some patients limit its application. Polyethylene glycol-conjugated asparaginase (PEG-ASP) has a lower immunogenicity and is a standard treatment in all pediatric acute lymphoblastic leukemia (ALL). In this study, we investigated the efficacy and toxicity of PEG-ASP instead of L-ASP as used in the BFM-90 regimen (PEG-ASP-BFM-90) for adult LBL.
Methods: Between June 2012 and July 2015, we treated 30 adult patients with newly diagnosed LBL, using PEG-ASP-BFM-90 in a prospective, multicenter and single-arm clinical study at 5 participating institutions in China.
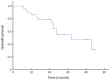
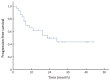
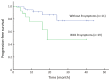
Results: All the 30 patients, including 19 males and 11 females with a median age of 30 (range: 18-62) years, completed 128 times of the PEG-ASP, with the median of 4 (range: 2-6) times. Patients did not receive radiotherapy at this time. The overall response rate was 86.7% (26/30), with 50.0% (15/30) complete response and 36.7% (11/30) partial response. The 3-year overall survival was 46.0% [95% confidence interval (95% CI), 28.2%-64.8%], and the 3-year progression-free survival was 43.0% (95% CI, 25.7%-62.0%). Major adverse events were myelosuppression, reduced fibrinogen, liver dysfunction and digestive tract toxicities. No allergic reaction and no treatment-related mortality or severe complications were recorded.
Conclusions: Our clinical data and observed outcomes indicate that 1 dose of PEG-ASP can replace multiple doses of native L-ASP in BFM-90, with predominantly grade 3-4 neutropenia for adult LBL, and no therapy-related deaths. The effect is similar to previous reports of PEG-ASP-containing regimens for adult ALL. Major advantages include less serious allergic reactions, 2-3 weeks of action duration, and convenience for patients and physicians.
Keywords: PEG-asparaginase; lymphoblastic lymphoma; treatment.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

Similar articles
-
PEG-asparaginase treatment regimens for acute lymphoblastic leukaemia in children: a network meta-analysis.Cochrane Database Syst Rev. 2023 May 31;5(5):CD014570. doi: 10.1002/14651858.CD014570.pub2. Cochrane Database Syst Rev. 2023. PMID: 37260073 Free PMC article.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Gemcitabine-based chemotherapy for advanced biliary tract carcinomas.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD011746. doi: 10.1002/14651858.CD011746.pub2. Cochrane Database Syst Rev. 2018. PMID: 29624208 Free PMC article.
Cited by
-
Comprehensive view on genetic features, therapeutic modalities and prognostic models in adult T-cell lymphoblastic lymphoma.Blood Sci. 2022 Jul 6;4(3):155-160. doi: 10.1097/BS9.0000000000000114. eCollection 2022 Jul. Blood Sci. 2022. PMID: 36518593 Free PMC article. Review.
-
Development and Validation of a Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry Method for the Determination of Asparagine in Human Serum.Int J Anal Chem. 2020 Feb 28;2020:6980392. doi: 10.1155/2020/6980392. eCollection 2020. Int J Anal Chem. 2020. PMID: 32180807 Free PMC article.
-
Allergic reactions to asparaginase: retrospective cohort study in pediatric patients with acute lymphoid leukemia.Hematol Transfus Cell Ther. 2021 Jan-Mar;43(1):9-14. doi: 10.1016/j.htct.2019.10.007. Epub 2020 Jan 22. Hematol Transfus Cell Ther. 2021. PMID: 32014473 Free PMC article.
-
Prognostic Value of Heterogeneity Index Derived from Baseline 18F-FDG PET/CT in Mantle Cell Lymphoma.Front Oncol. 2022 Apr 14;12:862473. doi: 10.3389/fonc.2022.862473. eCollection 2022. Front Oncol. 2022. PMID: 35494037 Free PMC article.
-
Phase II Study of Gemcitabine, Peg-Asparaginase, Dexamethasone and Methotrexate Regimen for Newly Diagnosed Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Final Analysis With Long-Term Follow-Up and Rational Research for the Combination.Front Oncol. 2022 Jan 24;12:796738. doi: 10.3389/fonc.2022.796738. eCollection 2022. Front Oncol. 2022. PMID: 35141162 Free PMC article.
References
-
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008.
-
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–9. - PubMed
-
- Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;15(104):1624–30. - PubMed
-
- Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4, 638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138:429–34. - PubMed
-
- Chen YC, Ho CL, Kao WY, et al. Adult lymphoblastic lymphoma in Taiwan: an analysis of treatment results of 26 patients. Ann Hematol. 2001;80:647–52. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
