Roles and Modalities of Ectonucleotidases in Remodeling the Multiple Myeloma Niche
- PMID: 28373875
- PMCID: PMC5357780
- DOI: 10.3389/fimmu.2017.00305
Roles and Modalities of Ectonucleotidases in Remodeling the Multiple Myeloma Niche
Abstract
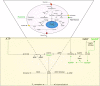
Ectoenzymes are cell surface molecules, which represent functional bridges between the environment and the cytoplasm. One set of ectoenzymes-CD39, CD38, CD203a, and CD73-leads to the generation of adenosine (ADO) by metabolizing ATP and NAD+. While ADO is known to control inflammation and suppress immune responses, other aspects of ADO function are still obscure, mainly due to its short half-life in biological fluids. Human multiple myeloma (MM) grows in the closed system of the bone marrow (BM) niche representing an ideal setting for studying ectoenzymes and their products. Another source of information on ectoenzyme function may derive from in vivo results of anti-CD38 antibody therapy in MM. Current results, obtained from in vitro models and from preliminary in vivo findings, indicate that ectoenzymes produce ADO locally in the BM niche. Furthermore, MM cells release microvesicles (MV), which thanks to their molecular cargo and surface ectoenzymes may function as particulate communicators outside of the niche. During anti-CD38 antibody therapy, the MV carry therapeutic IgG, determining that the prevalent orientation of MV will be toward cells and tissues expressing receptors for the IgG Fc domain. The resulting picture is one where MM adopts an immune escape strategy based on reshaping the environmental niche. This adaptation is followed by actions of MV that are exerted in biological fluids and circulating immune cells. By coating FcRs+ cells, MV modify pericellular spaces, reproducing the metabolic halo generated by ectoenzymes within closed systems.
Keywords: CD38; adenosine; ectoenzymes; microvesicles; multiple myeloma therapy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

