Acarbose Accelerates Wound Healing via Akt/eNOS Signaling in db/db Mice
- PMID: 28373902
- PMCID: PMC5360971
- DOI: 10.1155/2017/7809581
Acarbose Accelerates Wound Healing via Akt/eNOS Signaling in db/db Mice
Abstract
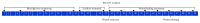
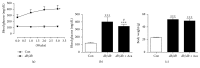
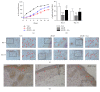
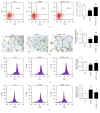
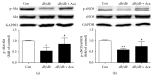
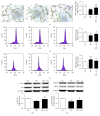
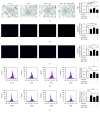
Refractory wound is a dreaded complication of diabetes and is highly correlated with EPC dysfunction caused by hyperglycemia. Acarbose is a widely used oral glucose-lowering drug exclusively for T2DM. Previous studies have suggested the beneficial effect of acarbose on improving endothelial dysfunction in patients with T2DM. However, no data have been reported on the beneficial efficacy of acarbose in wound healing impairment caused by diabetes. We herein investigated whether acarbose could improve wound healing in T2DM db/db mice and the possible mechanisms involved. Acarbose hastened wound healing and enhanced angiogenesis, accompanied by increased circulating EPC number in db/db mice. In vitro, a reversed BM-EPC dysfunction was observed after the administration of acarbose in db/db mice, as reflected by tube formation assay. In addition, a significantly increased NO production was also witnessed in BM-EPCs from acarbose treated db/db mice, with decreased O2 levels. Akt inhibitor could abolish the beneficial effect of acarbose on high glucose induced EPC dysfunction in vitro, accompanied by reduced eNOS activation. Acarbose displayed potential effect in promoting wound healing and improving angiogenesis in T2DM mice, which was possibly related to the Akt/eNOS signaling pathway.
Figures
Similar articles
-
Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice.Cardiovasc Diabetol. 2016 Jun 18;15:88. doi: 10.1186/s12933-016-0408-3. Cardiovasc Diabetol. 2016. PMID: 27316923 Free PMC article.
-
Metformin accelerates wound healing in type 2 diabetic db/db mice.Mol Med Rep. 2017 Dec;16(6):8691-8698. doi: 10.3892/mmr.2017.7707. Epub 2017 Oct 4. Mol Med Rep. 2017. PMID: 28990070 Free PMC article.
-
TLQP-21 facilitates diabetic wound healing by inducing angiogenesis through alleviating high glucose-induced injuries on endothelial progenitor cells.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):4993-5004. doi: 10.1007/s00210-023-02808-8. Epub 2024 Jan 6. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38183447 Free PMC article.
-
Nifedipine prevents vascular endothelial dysfunction in a mouse model of obesity and type 2 diabetes, by improving eNOS dysfunction and dephosphorylation.Biochem Biophys Res Commun. 2010 Dec 17;403(3-4):258-63. doi: 10.1016/j.bbrc.2010.11.008. Epub 2010 Nov 6. Biochem Biophys Res Commun. 2010. PMID: 21059340
-
Acarbose Monotherapy and Type 2 Diabetes Prevention in Eastern and Western Prediabetes: An Ethnicity-specific Meta-analysis.Clin Ther. 2015 Aug;37(8):1798-812. doi: 10.1016/j.clinthera.2015.05.504. Epub 2015 Jun 26. Clin Ther. 2015. PMID: 26118669 Review.
Cited by
-
Neutralization of excessive CCL28 improves wound healing in diabetic mice.Front Pharmacol. 2023 Jan 13;14:1087924. doi: 10.3389/fphar.2023.1087924. eCollection 2023. Front Pharmacol. 2023. PMID: 36713846 Free PMC article.
-
Resveratrol Attenuates Lipopolysaccharides (LPS)-Induced Inhibition of Osteoblast Differentiation in MC3T3-E1 Cells.Med Sci Monit. 2018 Apr 6;24:2045-2052. doi: 10.12659/msm.905703. Med Sci Monit. 2018. PMID: 29624568 Free PMC article.
-
Nicotinamide Riboside Enhances Endothelial Precursor Cell Function to Promote Refractory Wound Healing Through Mediating the Sirt1/AMPK Pathway.Front Pharmacol. 2021 May 12;12:671563. doi: 10.3389/fphar.2021.671563. eCollection 2021. Front Pharmacol. 2021. PMID: 34054544 Free PMC article.
-
[Effects of antihyperglycemics on endothelial progenitor cells].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Oct 25;49(5):629-636. doi: 10.3785/j.issn.1008-9292.2020.10.13. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 33210492 Free PMC article. Chinese.
-
Circulating exo-miR-154-5p regulates vascular dementia through endothelial progenitor cell-mediated angiogenesis.Front Cell Neurosci. 2022 Jul 29;16:881175. doi: 10.3389/fncel.2022.881175. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35966195 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

