Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray
- PMID: 28374016
- PMCID: PMC5370278
- DOI: 10.1523/ENEURO.0129-16.2017
Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray
Abstract
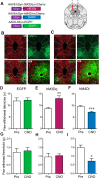
The ventrolateral periaqueductal gray (vlPAG) constitutes a major descending pain modulatory system and is a crucial site for opioid-induced analgesia. A number of previous studies have demonstrated that glutamate and GABA play critical opposing roles in nociceptive processing in the vlPAG. It has been suggested that glutamatergic neurotransmission exerts antinociceptive effects, whereas GABAergic neurotransmission exert pronociceptive effects on pain transmission, through descending pathways. The inability to exclusively manipulate subpopulations of neurons in the PAG has prevented direct testing of this hypothesis. Here, we demonstrate the different contributions of genetically defined glutamatergic and GABAergic vlPAG neurons in nociceptive processing by employing cell type-specific chemogenetic approaches in mice. Global chemogenetic manipulation of vlPAG neuronal activity suggests that vlPAG neural circuits exert tonic suppression of nociception, consistent with previous pharmacological and electrophysiological studies. However, selective modulation of GABAergic or glutamatergic neurons demonstrates an inverse regulation of nociceptive behaviors by these cell populations. Selective chemogenetic activation of glutamatergic neurons, or inhibition of GABAergic neurons, in vlPAG suppresses nociception. In contrast, inhibition of glutamatergic neurons, or activation of GABAergic neurons, in vlPAG facilitates nociception. Our findings provide direct experimental support for a model in which excitatory and inhibitory neurons in the PAG bidirectionally modulate nociception.
Keywords: DREADDs; Descending modulation; PAG; RVM; chemogenetics; pain.
Figures




References
-
- Antal M, Odeh F (1998) The projections of the midbrain periaqueductal gray to serotonergic and noradrenergic nuclei of the pons and medulla oblongata in the rat. Eur J Neurosci 10:218–218. - PubMed
-
- Bandler R, Keay KA (1996) Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 107:285–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
