Alternative splicing: the pledge, the turn, and the prestige : The key role of alternative splicing in human biological systems
- PMID: 28374191
- PMCID: PMC5602094
- DOI: 10.1007/s00439-017-1790-y
Alternative splicing: the pledge, the turn, and the prestige : The key role of alternative splicing in human biological systems
Abstract
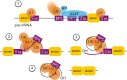
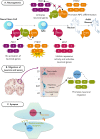
Alternative pre-mRNA splicing is a tightly controlled process conducted by the spliceosome, with the assistance of several regulators, resulting in the expression of different transcript isoforms from the same gene and increasing both transcriptome and proteome complexity. The differences between alternative isoforms may be subtle but enough to change the function or localization of the translated proteins. A fine control of the isoform balance is, therefore, needed throughout developmental stages and adult tissues or physiological conditions and it does not come as a surprise that several diseases are caused by its deregulation. In this review, we aim to bring the splicing machinery on stage and raise the curtain on its mechanisms and regulation throughout several systems and tissues of the human body, from neurodevelopment to the interactions with the human microbiome. We discuss, on one hand, the essential role of alternative splicing in assuring tissue function, diversity, and swiftness of response in these systems or tissues, and on the other hand, what goes wrong when its regulatory mechanisms fail. We also focus on the possibilities that splicing modulation therapies open for the future of personalized medicine, along with the leading techniques in this field. The final act of the spliceosome, however, is yet to be fully revealed, as more knowledge is needed regarding the complex regulatory network that coordinates alternative splicing and how its dysfunction leads to disease.
Keywords: Alternative Splice; Duchenne Muscular Dystrophy; Round Spermatid; Spinal Muscular Atrophy; Splice Factor.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures






References
-
- Abbas AK, Lichtman AHH, Pillai S (2014) Cellular and molecular immunology. Elsevier Health Sciences
-
- Ahmadi Rastegar D, Sharifi Tabar M, Alikhani M, et al. Isoform-level gene expression profiles of human Y chromosome azoospermia factor genes and their X chromosome paralogs in the testicular tissue of non-obstructive azoospermia patients. J Proteome Res. 2015;14:3595–3605. doi: 10.1021/acs.jproteome.5b00520. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Installation Grant (3057)/European Molecular Biology Organization/International
- Investigador FCT Starting Grant (IF/00595/2014)/Fundação para a Ciência e a Tecnologia/International
- PhD fellowship (PD/BD/105854/2014)/Fundação para a Ciência e a Tecnologia/International
- PhD fellowship (PD/BD/128283/2017)/Fundação para a Ciência e a Tecnologia/International
- Women in Science Program (ERI/NCS/FLP/CDC.13.94)/United Nations Educational, Scientific and Cultural Organization/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

