Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse
- PMID: 28374413
- PMCID: PMC5491865
- DOI: 10.1113/JP274030
Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse
Abstract
Key points: Leptin, is a 16 kDa pleiotropic peptide not only primarily secreted by adipocytes, but also produced by other tissues, including the heart. Controversy exists regarding the adverse and beneficial effects of leptin on the heart We analysed the effect of a non-hypertensive dose of leptin on cardiac function, [Ca2+ ]i handling and cellular electrophysiology, which participate in the genesis of pump failure and related arrhythmias, both in control mice and in mice subjected to chronic pressure-overload by transverse aorta constriction. We find that leptin activates mechanisms that contribute to cardiac dysfunction under physiological conditions. However, after the establishment of pressure overload, an increase in leptin levels has protective cardiac effects with respect to rescuing the cellular heart failure phenotype. These beneficial effects of leptin involve restoration of action potential duration via normalization of transient outward potassium current and sarcoplasmic reticulum Ca2+ content via rescue of control sarcoplasmic/endoplasmic reticulum Ca2+ ATPase levels and ryanodine receptor function modulation, leading to normalization of Ca2+ handling parameters.
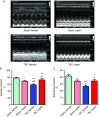
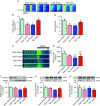
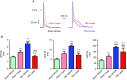
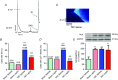
Abstract: Leptin, is a 16 kDa pleiotropic peptide not only primary secreted by adipocytes, but also produced by other tissues, including the heart. Evidence indicates that leptin may have either adverse or beneficial effects on the heart. To obtain further insights, in the present study, we analysed the effect of leptin treatment on cardiac function, [Ca2+ ]i handling and cellular electrophysiology, which participate in the genesis of pump failure and related arrhythmias, both in control mice and in mice subjected to chronic pressure-overload by transverse aorta constriction (TAC). Three weeks after surgery, animals received either leptin (0.36 mg kg-1 day-1 ) or vehicle via osmotic minipumps for 3 weeks. Echocardiographic measurements showed that, although leptin treatment was deleterious on cardiac function in sham, leptin had a cardioprotective effect following TAC. [Ca2+ ]i transient in cardiomyocytes followed similar pattern. Patch clamp experiments showed prolongation of action potential duration (APD) in TAC and leptin-treated sham animals, whereas, following TAC, leptin reduced the APD towards control values. APD variations were associated with decreased transient outward potassium current and Kv4.2 and KChIP2 protein expression. TAC myocytes showed a higher incidence of triggered activities and spontaneous Ca2+ waves. These proarrhythmic manifestations, related to Ca2+ /calmodulin-dependent protein kinase II and ryanodine receptor phosphorylation, were reduced by leptin. The results of the present study demonstrate that, although leptin treatment was deleterious on cardiac function in control animals, leptin had a cardioprotective effect following TAC, normalizing cardiac function and reducing arrhythmogeneity at the cellular level.
Keywords: heart failure; intracellular calcium; leptin.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures

Similar articles
-
Prolonged leptin treatment increases transient outward K⁺ current via upregulation of Kv4.2 and Kv4.3 channel subunits in adult rat ventricular myocytes.Pflugers Arch. 2014 May;466(5):903-14. doi: 10.1007/s00424-013-1348-3. Epub 2013 Sep 18. Pflugers Arch. 2014. PMID: 24046152
-
Ca2+/calmodulin-dependent protein kinase II is essential in hyperacute pressure overload.J Mol Cell Cardiol. 2020 Jan;138:212-221. doi: 10.1016/j.yjmcc.2019.12.002. Epub 2019 Dec 10. J Mol Cell Cardiol. 2020. PMID: 31836540
-
Calcium/Calmodulin Protein Kinase II-Dependent Ryanodine Receptor Phosphorylation Mediates Cardiac Contractile Dysfunction Associated With Sepsis.Crit Care Med. 2017 Apr;45(4):e399-e408. doi: 10.1097/CCM.0000000000002101. Crit Care Med. 2017. PMID: 27648519
-
The potential role of Kv4.3 K+ channel in heart hypertrophy.Channels (Austin). 2014;8(3):203-9. doi: 10.4161/chan.28972. Channels (Austin). 2014. PMID: 24762397 Free PMC article. Review.
-
The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure.Cardiovasc Res. 1998 Feb;37(2):312-23. doi: 10.1016/s0008-6363(97)00256-3. Cardiovasc Res. 1998. PMID: 9614488 Review.
Cited by
-
A Neural Circuit Mechanism Controlling Breathing by Leptin in the Nucleus Tractus Solitarii.Neurosci Bull. 2022 Feb;38(2):149-165. doi: 10.1007/s12264-021-00742-4. Epub 2021 Jul 2. Neurosci Bull. 2022. PMID: 34212297 Free PMC article.
-
Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts.J Clin Med. 2025 May 7;14(9):3250. doi: 10.3390/jcm14093250. J Clin Med. 2025. PMID: 40364280 Free PMC article. Review.
-
Beneficial effects of paricalcitol on cardiac dysfunction and remodelling in a model of established heart failure.Br J Pharmacol. 2020 Jul;177(14):3273-3290. doi: 10.1111/bph.15048. Epub 2020 Apr 22. Br J Pharmacol. 2020. PMID: 32154912 Free PMC article.
-
MCC950 ameliorates ventricular arrhythmia vulnerability induced by heart failure.Bioengineered. 2022 Apr;13(4):8593-8604. doi: 10.1080/21655979.2022.2053813. Bioengineered. 2022. PMID: 35287557 Free PMC article.
-
Leptin Levels are Associated with Subclinical Cardiac Dysfunction in Obese Adolescents.Diabetes Metab Syndr Obes. 2020 Mar 27;13:925-933. doi: 10.2147/DMSO.S245048. eCollection 2020. Diabetes Metab Syndr Obes. 2020. PMID: 32273744 Free PMC article.
References
-
- Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A & Hasegawa K (2007). Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol 292, H2387–H2396. - PubMed
-
- Ahrén B, Larsson H, Wilhelmsson C, Näsman B & Olsson T (1997). Regulation of circulating leptin in humans. Endocrine 7, 1–8. - PubMed
-
- Atkinson LL, Fischer MA & Lopaschuk GD (2002). Leptin activates cardiac fatty acid oxidation independent of changes in the AMP‐activated protein kinase‐acetyl‐CoA carboxylase‐malonyl‐CoA axis. J Biol Chem 277, 29424–29430. - PubMed
-
- Baartscheer A, Schumacher CA, Belterman CN, Coronel R & Fiolet JW (2003). [Na+]i and the driving force of the Na+/Ca2+‐exchanger in heart failure. Cardiovasc Res 57, 986–995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

